
Novel accelerated corrosion test for LY12CZ and LC4CS aluminum alloys
CAI Jian-ping(蔡健平), LIU Ming(刘 明)
Beijing Institute of Aeronautic Materials, Beijing 100095, China
Received 28 July 2006; accepted 15 September 2006
Abstract:
A new accelerated corrosion test—comprehensive environmental test (CET) was developed in order to estimate the outdoor corrosion of aluminum alloys in marine environment. The environmental characteristics in CET were studied by atmospheric corrosion monitor (ACM), and the morphology of corrosion product was observed by SEM. The correlation between the accelerated corrosion tests and outdoor exposure was discussed. The results show that the anti-corrosion ranking for LY12CZ, LC4CS, clad LY12CZ, and clad LC4CS in CET is the same as that of the alloys exposed outdoor, and ACM study shows that CET demonstrates the same environmental characteristics as that exposed outdoor. CET is a more accurate accelerated corrosion test, and a mathematical relation was obtained to describe the relation between CET and outdoor test.
Keywords: LY12CZ; LC4CS; accelerated corrosion test; comprehensive environmental test; marine environment
1 Introduction
Accelerated corrosion tests are necessary for development and qualification of materials. At present, salt spray test is widely used in aeronautical industry. For instance, WU et al [1] carried out salt spray test for 7075 aluminum and proposed concept of corrosion damage, by which a liner relation between accelerated corrosion test and actual exposure can be obtained. However, salt spray test does not consider the corrosion process of materials and the unrealistic simulation of exterior environment is a serious shortcoming. BALDWIN et al [2] pointed out that alternate immersion test appears to be more accurate than standard salt spray testing since alternate immersion test simulates wet/dry cycles in actual atmospheric environment. So some complex corrosion tests were proposed [3-5]. LIU et al [6] studied atmospheric corrosion of LC4CS aluminum alloy by periodic rain tests using an artificial climatic complex tester [3], but the study only focused on an individual material, and did not compare corrosion property of different aluminum alloys. It is doubt that the test can be used for searching “better” material.
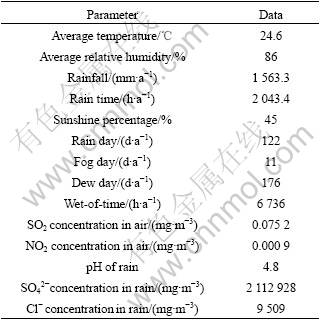
Here a new accelerated corrosion test based on outdoor exposure result of LC4CS and LY12CZ is designed. The characteristics of test environment are illustrated by atmospheric corrosion monitoring (ACM), and anti-corrosion ranking of aluminum alloys is also compared. It is found that there may be a non-linear relation between the accelerated corrosion test and outdoor exposure. Since corrosion is a serious problem for aluminum alloys in marine environment of South China (Wanning city in Table 1), this accelerated corrosion test may be used to screen better anti-corrosion materials and estimate service life of new developed 2××× and 7××× series aluminum alloys.
Table 1 Environmental data in Wanning city
2 Experimental
2.1 Specimens and materials
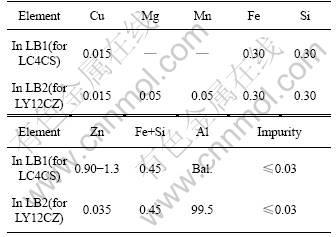
Specimens are 100 mm×50 mm×(2-3) mm coupons, the materials of coupons are commercial aeronautic 2××× series aluminum alloys of clad LY12CZ, LY12CZ and 7××× series aluminum of clad LC4CS, LC4CS. The compositions of LY12CZ and LC4CS are listed in Tables 2 and 3, respectively. The compositions of cladding layers are listed in Table 4. The specimens are degreased with ethanol, washed by distilled water and then dried before accelerated tests.
Table 2 Chemical composition of LY12CZ alloy (mass fraction, %)
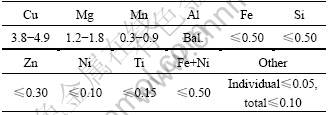
Table 3 Chemical composition of LC4CS alloy (mass fraction, %)

Table 4 Chemical compositions of clad LC4CS and clad LY12CZ (mass fraction, %)
2.2 Accelerated corrosion tests
Continuous salt spray test was carried out by Q-Fog salt spray chamber according to ASTM B117. The salt solution was 5% NaCl; temperature was 35 ℃; the deposition rate of salt fog was 1-2 mL/h; and pH was 6.5-7.2.
The alternating immersion test was performed by FL test chamber. The specimens were placed on a Ferris wheel arrangement. The rotating rate was 6(?)/min, and thereby passes the specimens a stationary tank of solution. So in each cycle specimens underwent 10 min immersion in solution and then dried for 50 min in air. The immersion solution was 3.5% NaCl (pH 6.5-7.2), and temperature of solution and air was 35 ℃.
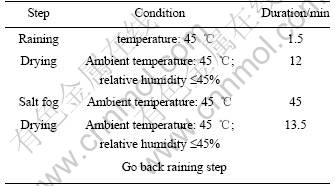
Comprehensive environmental test was carried out by CET-2000 tester [5]. The each test cycle was composed by raining+ drying +salt spray +drying period. The rainwater was 4% NaCl +0.8%Na2S2O8 +0.05% NaNO3 (pH: 4-5), and during the raining, the surface of specimens was in completely wet condition; salt spray solution was 3.5% NaCl (pH: 6.5-7.2). Table 5 lists the test condition of CET.
Table 5 Testing conditions of comprehensive environmental test
2.3 Environment monitoring
Atmospheric corrosion monitor (ACM) is a galvanic cell of different metals encapsulated in epoxy resin, and it was often used to characterize the corrosivity of outdoor exposure site[7]. Here CMB-1512 ACM instrument, which is composed by Fe/Zn galvanic cell, was used to monitor corrosion environment. During the test, the galvanic current curve measured was recorded.
2.4 Morphology of corrosion product
The morphology of corroded surface in accelerated corrosion test was observed by JSM-5600LV scanning electron microscopy (SEM).
2.5 Corrosion kinetics
During accelerated corrosion tests, specimens were removed from test chamber, and then the corrosion product was removed in solution of 80 g/L CrO3+200 mL/L H3PO4, and mass loss due to corrosion was calculated. So the relation between corrosion mass and test time can be obtained.
3 Results and discussion
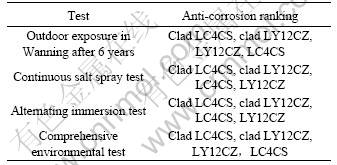
3.1 Anti-corrosion ranking in accelerated corrosion test and outdoor exposure
Table 6 lists the anti-corrosion ranking for aluminum alloys during accelerated corrosion test and outdoor exposure in Wanning city. Table 6 lists the anti-corrosion ranking in continuous salt spray and alternating immersion test that are different from those in outdoor exposure, and only comprehensive environmental test repeated the anti-corrosion ranking in outdoor exposure. This result demonstrates that continuous salt spray and alternating immersion test can not “recognize” better anti-corrosion materials, but CET test gives correct information about anti-corrosion ranking. So CET test is a more accurate accelerated corrosion test.
Table 6 Anti-corrosion ranking in accelerated corrosion test and outdoor exposure
3.2 Environmental characteristics in CET test
Early research found that the galvanic current curve of ACM in outdoor exposure showed pulse current shape and in the curve there were large, medium and small current period, which corresponds to raining, fog/dew and dry condition respectively. Fig.1 shows a typical galvanic current curve obtained in CET test. It can also be seen that the curve has similar characteristics to that of exposed outdoor test. The sharp current peaks correspond to raining, the medium current peaks are caused by fog, and the current troughs appear during dry period. So the CET gives simulation of wet, damp and dry atmospheric corrosion [7]. Consequently, CET test gives more realistic simulation of exterior environment, and corrosion process of materials.
3.3 Morphology
Fig.2 shows the morphologies of LC4CS and LY12CZ in CET test. In Fig.2, the surface of corrosion product has many cracks, which is similar to those of specimens after exposed outdoor [8].
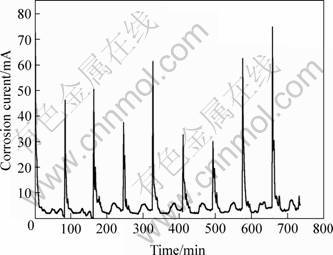
Fig.1 Typical galvanic current curve measured by ACM in CET test
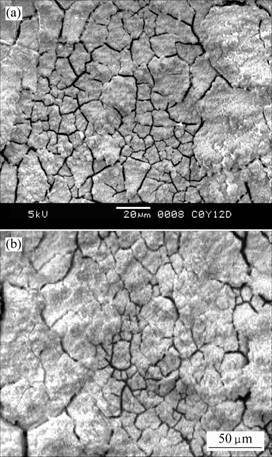
Fig.2 SEM images of aluminum alloys in CET LC4CS after 288 h (a) and LY12CZ after 288 h (b)
3.4 Mathematical relation between CET and outdoor exposure
The mass loss—time regression equation in CET and outdoor exposure is shown in Table 7. Table 7 illustrates that the corrosion kinetics in CET and outdoor exposure follow bilogarithm relation.
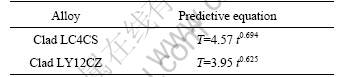
From kinetics equation of Table 7, the predictive equations are obtained and listed in Table 8. The predictive equation can be used to estimate outdoor corrosion, for example, the first day CET can produce corrosion of 4.57 year exposure in Wanning for clad LC4CS. Table 8 also demonstrates that the correlation between the accelerated test and outdoor exposure is not linear, which means that rate of accelerated corrosion test changes with test time.
Table 7 Bi-logarithm regression equations for accelerated and outdoor tests
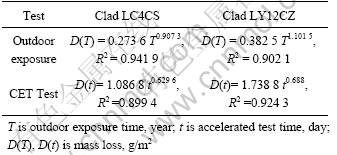
Table 8 Predictive equations for Wanning atmospheric corrosion from CET test
4 Conclusions
1) A comprehensive environmental test, which included rain/fog/dry cycles, was developed to simulate an actual atmospheric corrosion.
2) The comprehensive environmental test provides accurate simulation to actual exposure condition. It reproduced the anti-corrosion ranking of studied aluminum alloys completely.
3) A mathematical equation was obtained to describe the relationship between test result in CET and that in outdoor exposure.
References
[1] WU Ling, SUN Qin, GUO Ying-nan. Accelerated corrosion tests of high strength 7075 alloy in salt water spray test [J]. Journal of Mechanical Strength, 2006, 28(1): 138-140. (in Chinese)
[2] BALDWIN K R, SMITH C J E. Accelerated corrosion test for aerospace materials: Current limitations and future trend air craft [J]. Engineering and Aerospace Technology, 1999, 71(3): 239-244.
[3] LI J, LI M, SUN Z. Development of and artificial climatic complex accelerated corrosion tester and investigation of complex accelerated corrosion test method [J]. Corrosion, 1999, 55(5): 498-502.
[4] HAROLD D. Hilton select a cyclic corrosion test cabinet [J]. Materials Performance, 2003, 42(2): 76-79.
[5] CAI Jian-ping, TANG Zhi-hui, ZHANG Xiao-yun, SUN Zhi-hua, LIU Ming, WANG Yong-zhe, LI Mu-zheng. CET-2000 comprehensive environmental tester and its application[J]. Corrosion Science and Technology, 2004, 16(6): 411-412. (in Chinese)
[6] LIU Ming, ZHANG Xiao-yun, LU Feng, TAO Chun-hu, WANG Yong-zhe, CAI Jian-ping, LI Mu-zheng. Accelerated tests for simulation of atmospheric corrosion of LC4CS aluminum alloy [J]. Corrosion Science and Technology, 2005, 17(4): 271-274. (in Chinese)
[7] CAO Chun-nan. Atmospheric Environment and its Corrosivity, Natural Environmental Corrosion of Chinese Materials[M]. Beijing: Chemical Industry Press, 2005: 69-89. (in Chinese)
[8] CAO Chun-nan. Atmospheric Corrosion of Non-ferrous Metals, Natural Environmental Corrosion of Chinese Materials[M]. Beijing: Chemical Industry Press, 2005: 108-111. (in Chinese)
(Edited by LONG Huai-zhong)
Foundation item: Project(G1999065004) supported by the National Key Basic Research Program of China
Corresponding author: CAI Jian-ping; Tel: +86-10-62496445; E-mail: JP.Cai@BIAM.ac.cn


