
Microstructure and electrochemical performances of LiF-coated spinel LiMn2O4
BAI Ying(白 莹)1, 2, WU Chuan(吴 川)1, 2, WU Feng(吴 锋)1, 2
1. School of Chemical Engineering and Environment, Beijing Institute of Technology, Beijing 100081, China;
2. National Development Center for High Technology Green Materials, Beijing 100081, China
Received 15 July 2007; accepted 10 September 2007
Abstract:
LiF-coated LiMn2O4 samples were prepared via a chemical method. X-ray diffraction (XRD) patterns show that the bare LiMn2O4 and the LiF-coated LiMn2O4 samples are all spinel structure in ![]() space group. The apparent morphologies, the spectroscopic properties and the LiF distributions of the as-prepared samples were studied by scanning electronic microscopy (SEM), Fourier infrared spectroscopy (FTIR), transmission electronic microscopy (TEM), selected area electron diffractometry (SAED) respectively. The LiF-coated LiMn2O4 gets a more stable surface than bare LiMn2O4, and changes the interaction between the cathode material and the electrolyte. Therefore, it can endure overcharge in the secondary lithium batteries, and achieve better electrochemical performances even when charged to 4.7 V and 4.9 V.
space group. The apparent morphologies, the spectroscopic properties and the LiF distributions of the as-prepared samples were studied by scanning electronic microscopy (SEM), Fourier infrared spectroscopy (FTIR), transmission electronic microscopy (TEM), selected area electron diffractometry (SAED) respectively. The LiF-coated LiMn2O4 gets a more stable surface than bare LiMn2O4, and changes the interaction between the cathode material and the electrolyte. Therefore, it can endure overcharge in the secondary lithium batteries, and achieve better electrochemical performances even when charged to 4.7 V and 4.9 V.
Key words:
spinel LiMn2O4; secondary lithium batteries; surface modification; LiF-coating;
1 Introduction
Spinel LiMn2O4 is a widely studied cathode material for secondary lithium batteries. However, there are some barriers on the way to extensive applications of this material, among which Jahn-Teller effect, Mn dissolution and electrolyte decomposition are the major ones. To improve the structural stability and electrochemical performances of spinel LiMn2O4, many attentions are paid to cation-doping[1-7], or anion-doping[8-11]. Recently, it is found that surface modification can improve the electrochemical performances without change the body phase of spinel LiMn2O4 heavily. Both chemical modification methods[12-13] and electrochemical modification methods[14] are proved effective.
It is well known that a passivating film will attach on the electrodes in a secondary lithium battery upon cycling. Additionally, the stable inorganic components in the passivating films are LiF, LiCO3, LiOH, etc. Therefore, it is promising to adopt these chemicals to modify LiMn2O4 surface, and suppress the corrosion from the electrolyte.
In this study, a commercial spinel LiMn2O4 is selected as the raw material, LiF is coated on LiMn2O4 surface via a chemical method. The structures and the electrochemical performances of the bare LiMn2O4 and the LiF-coated LiMn2O4 are studied and compared in detail.
2 Experimental
2.1 Sample preparation
A commercial spinel LiMn2O4 (Cellseed M) was selected as the bare LiMn2O4. To get LiF-coated spinel LiMn2O4, 200 mL LiOH solution with a concentration of 0.1 mol/L was first placed into a beaker and then 5 g bare LiMn2O4 was introduced in and stirred thoroughly with the LiOH solution. Subsequently, a 0.1 mol/L KF solution was dropped into the beaker with sustaining stiring. LiF particles were formed and adhered on the LiMn2O4, and the nominal ratio of LiF to LiMn2O4 was fixed as 5?95. The precursor of LiF-coated spinelLiMn2O4 was washed with distilled water, and then vacuum-dried at 100 ℃. Finally, the precursor was heated at 600 ℃ for 2 h, and cooled to ambient temperature. Thus, LiF-coated spinel LiMn2O4 was prepared by this way.
2.2 Structural characterization
XRD patterns of the as-prepared samples were recorded on a Rigaku B/max-2400 X-ray diffraction meter with Cu Kα radiation. The scanning range was from 10? to 100?, and the scanning rate was 8 ℃/min in steps of 0.02?. The apparent morphologies of the as-prepared samples were observed on a JSM-6301 scanning electron microscope (SEM), and the microcosmic structures were observed by a JEOL 200CX transmission electron microscope (TEM). The FTIR spectra are the average of 100 scans obtained on an FTS-60 V spectrometer (BIO-RAD) with a resolution of 4 cm-1.
2.3 Electrochemical tests
The electrochemical performances were tested by adopting Swagelok-type secondary lithium batteries assembled in an argon-filled glove box (MBRAUN), where metal lithium foils served as counter and reference electrodes, and 1 mol/L LiPF6 in a 1?1(volume fraction) mixture of ethylene carbonate (EC) and dimethyl carbonate (DMC) as electrolyte. A sheet of Celgard? 2300 was used as a separator. The cathodes were prepared by coating slurries of spinel LiMn2O4 powder, carbon black and cyclopentanone-dissolved polyvinylidene fluoride (PVDF) onto aluminum foils, and the resultant mass ratio of LiMn2O4, carbon black, and PVDF was 85?10?5. Subsequently, the films were vacuum-dried at 55 ℃ for 24 h, compressed between two stainless steel plates, and then cut into sheets with an area of 0.5 cm2. Cyclic voltammetry was carried out on a CHI660 electrochemical work station, and the charge-discharge test was performed on a LAND- CT2001A battery tester.
3 Results and discussion3.1 XRD patterns
It is found that LiF-coating does not change the body structure of spinel, all the samples show spinel phases with the structure of ![]() space group, as shown in Fig.1. Since LiF content is very low in the samples, no distinct LiF diffractions are detected. The lattice constants of bare LiMn2O4 is 8.220 6 ?, whereas that of the LiF-coated LiMn2O4 after heat-treatment at 600 ℃ is 8.219 6 ?, which indicates that the diffusion of small amount of LiF into LiMn2O4 body phase helps the metal-oxygen bonds to shrink.
space group, as shown in Fig.1. Since LiF content is very low in the samples, no distinct LiF diffractions are detected. The lattice constants of bare LiMn2O4 is 8.220 6 ?, whereas that of the LiF-coated LiMn2O4 after heat-treatment at 600 ℃ is 8.219 6 ?, which indicates that the diffusion of small amount of LiF into LiMn2O4 body phase helps the metal-oxygen bonds to shrink.
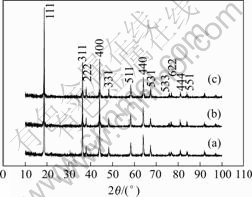
Fig.1 XRD patterns of LiF-coated spinel LiMn2O4: (a) Bare LiMn2O4; (b) LiF-coated spinel LiMn2O4 before heat-treating; (c) LiF-coated spinel LiMn2O4 after heat-treating for 2 h at 600 ℃
3.2 SEM images
Fig.2(a) shows the SEM image of bare LiMn2O4, which shows polyhedron particles in the sizes from 100 nm to 300 nm. After LiF deposition on bare LiMn2O4 particles, the apparent morphologies of the LiMn2O4 particles show a wider size distribution, see Fig.2(b). After treated at 600 ℃, the particles of LiF-coated LiMn2O4 distribute uniformly as shown in Fig.2(c).
3.3 FTIR analysis
According to previous study[15], the FTIR absorptions of the samples here are attributed to the asymmetric stretching modes of the Mn—O bands, as shown in Fig.3. For bare LiMn2O4, the vibrations of the Mn—O bands are located at 615 cm-1 and 512 cm-1, respectively. While the vibrations of the Mn—O bands for LiF-coated LiMn2O4 are located at 620 cm-1 and 513 cm-1, respectively.
After treated at 600 ℃, some of the LiF may diffuse into the body phase of LiMn2O4; the additional fluorine ions will give a diversity of the anions, and disturb the interaction between the Mn—anion bonds, and lead to an obvious shift on the vibration bands. Therefore, it is evident that the Mn—O stretching band at 615 cm-1 has a blue shift to 620 cm-1.
3.4 TEM image and SAED spectrum
Fig.4 gives an assured evidence for the existence of LiF on the surface of LiMn2O4. Fig.4(a) shows the TEM image of LiF-coated LiMn2O4 heat-treated at 600 ℃, Fig.4(b) shows the SAED spectrum for the selected area marked by the white arrow in the TEM image. The d value of the dark ring in the SAED spectrum is the same as LiF (200) diffraction, indicating that there are LiF particles on LiMn2O4 surface, and has a discrete distribution.
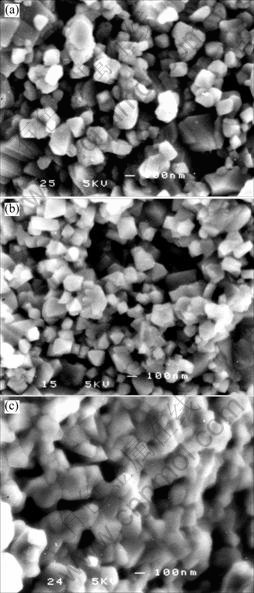
Fig.2 SEM images of LiF-coated spinel LiMn2O4: (a) Bare LiMn2O4; (b) LiF-coated spinel LiMn2O4 before heat-treating; (c) LiF-coated spinel LiMn2O4 after heat-treating for 2 h at 600 ℃
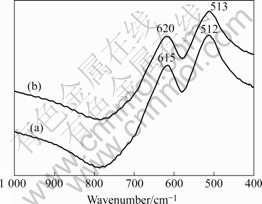
Fig.3 FTIR spectra of LiF-coated spinel LiMn2O4: (a) Before heat-treating; (b) After heat-treating at 600 ℃ for 2 h
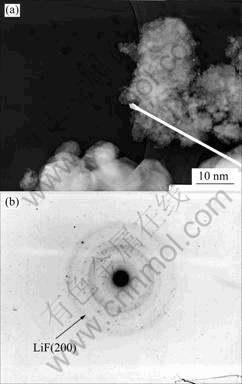
Fig.4 TEM image (a) and SAED spectrum (b) of LiF-coated spinel LiMn2O4 heat-treated at 600 ℃
4 Electrochemical performancesIn previous study[16], cyclic voltammetry result shows that an evident additional peak in the bare LiMn2O4 emerges at 4.9 V, which indicates the decomposition of electrolyte. In this study, since LiF is a stable component in the passivating films, the LiF particles on LiMn2O4 help to achieve a good LiF-coating, the 4.9 V peak of the cyclic voltammograms of LiF-coated spinel LiMn2O4 after heat-treated at 600 ℃ is more flat, as shown in Fig.5, which indicate that the decomposition of electrolyte and the corrosions of LiMn2O4 at high voltage are suppressed. Furthermore, the existence of coating layer on LiMn2O4 results in a heavy polarization in the initial cycle, as shown in Fig.5.
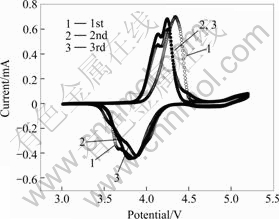
Fig.5 First three cyclic voltammograms of LiF-coated spinel LiMn2O4 heat-treated at 600 ℃
Generally, the charge cut-off voltage of spinel LiMn2O4 does not exceeds 4.3 V. However, since the secondary lithium batteries are often abused in practice, the experimental batteries here are cycled at a constant current density of 0.2 mA/cm2 in the voltage range of 3.0-4.7 V and 3.0-4.9 V, respectively, as shown in Figs.6(a) and (b). When cycled between 3.0-4.7 V, the LiF-coated LiMn2O4 has an initial capacity of 119 mA?h/g, which is almost the same as that of the bare LiMn2O4, 122 mA?h/g. However, the performances of the samples after cycling are quite different. At 25th cycle, the capacity of the bare LiMn2O4 has a decrease of 12%, and remains at only 107 mA?h/g; whereas the capacity of the LiF-coated LiMn2O4 only has a decrease of 4%, and remains at 114 mA?h/g.
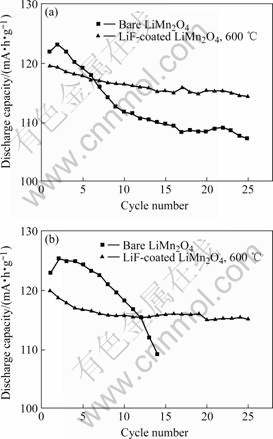
Fig.6 Discharge capacity as function of cycle number for LiF-coated spinel LiMn2O4 and bare LiMn2O4: (a) At 3.0-4.7 V; (b) At 3.0-4.9 V
When cycled between 3.0-4. 9V, the LiF-coated LiMn2O4 has an initial capacity of 120 mA?h/g, which is almost the same as that of the bare LiMn2O4, 123 mA?h/g. At 25th cycle, the capacity of the bare LiMn2O4 remains at only 109 mA?h/g, and has a tendency of sharp decrease. Whereas the capacity of the LiF-coated LiMn2O4 remains at 115 mA?h/g, and the reversible capacity is well kept in the following cycles. It implies that the LiF-coated LiMn2O4 has excellent endurance for overcharge in secondary lithium batteries.
5 Conclusions
1) The LiF-coated LiMn2O4 samples were prepared via a chemical method. XRD patterns and SEM images show that small amount of LiF-coating will not change the body structure and the apparent morphologies of LiMn2O4 evidently. However, FTIR spectra show that Mn—O stretching band has a blue shift after LiF-coating, and TEM tests indicate that LiF has a discrete distribution on LiMn2O4 surface.
2) The LiF-coated LiMn2O4 gets a more stable surface than bare LiMn2O4, and changes the interaction between the cathode material and the electrolyte. Therefore, it can endure overcharge in the secondary lithium batteries, and achieve better electrochemical performances under abused conditions.
References[1] WU Chuan, WU Feng, CHEN Li-quan, HUANG Xue-jie. X-ray diffraction and X-ray photoelectron spectroscopy analysis of Cr-doped spinel LiMn2O4 for lithium ion batteries[J]. Solid State Ionics, 2002, 152/153: 335-339.
[2] KAKUDA T, UEMATSU K, TODA K, SATO M, Electrochemical performance of Al-doped LiMn2O4 prepared by different methods in solid-state reaction[J]. J Power Sources, 2007, 167: 499-503.
[3] FU Y P, SU Y H, WU S H, LIN C H. LiMn2-yMyO4(M=Cr, Co) cathode materials synthesized by the microwave-induced combustion for lithium ion batteries[J]. J Alloys Comp, 2006, 426: 228-234.
[4] LIU Dong-qiang, HE Ze-zhen, LIU Xing-quan. Synthesis and characterization of LiGaxMn2-xO4 (0≤x≤0.05) by triethanolamine- assisted sol-gel method[J]. J Alloys Comp, 2007, 440: 69-73.
[5] PARK D H, LIM S T, HWANG S J, CHOY J H, CHOI J H, CHOO J. Influence of nickel content on the chemical bonding character of LiMn2-xNixO4 spinel oxides[J]. J Power Sources, 2006, 159: 1346-1352.
[6] CHO W, RA W, SHIRAKAWA J, NAKAYAMA M, WAKIHARA M. Synthesis and electrochemical properties of nonstoichiometric LiAlxMn2-xO4-d as cathode materials for rechargeable lithium ion battery[J]. J Solid State Chem, 2006, 179: 3534-3540.
[7] AURBACH D, MARKOVSKY B, SALITRA G, MARKEVICH E, TALYOSSEF Y, KOLTYPIN M, NAZAR L, ELLIS B, KOVACHEVA D. Review on electrode-electrolyte solution interactions, related to cathode materials for Li-ion batteries[J]. J Power Sources, 2007, 165: 491-499.
[8] STROBEL P, ANNE M, CHABRE Y, PALACIN M R, SEGUIN L, VAUGHAN G, AMATUCCI G, TARASCON J M. Characteristics of the 4 V plateau in LiMn2(O4-xFx) studied by in situ synchrotron X-ray diffraction[J]. J Power Sources, 1999, 81/82: 458-462.
[9] MOLENDA M, DZIEMBAJ R, MAJDA D, DUDEK M. Synthesis and characterisation of sulphided lithium manganese spinels LiMn2O4-ySy prepared by sol-gel method[J]. Solid State Ionics, 2005, 176: 1705-1709.
[10] SON J T, KIM H G. New investigation of fluorine-substituted spinel LiMn2O4-xFx by using sol-gel process[J]. J Power Sources, 2005, 147: 220-226.
[11] HE Xiang-ming, LI Jian-jun, CAI Yan, WANG Yao-wu, YING Jie-rong, JIANG Chang-yin, WAN Chun-rong. Fluorine doping of spherical spinel LiMn2O4[J]. Solid State Ionics, 2005, 176: 2571-2576.
[12] TU J, ZHAO X B, XIE J, CAO G S, ZHUANG D G, ZHU T J, Tu J P. Enhanced low voltage cycling stability of LiMn2O4 cathode by ZnO coating for lithium ion batteries[J]. J Alloys Comp, 2007, 432: 313-317.
[13] HA H W, YUN N J, KIM K. Improvement of electrochemical stability of LiMn2O4 by CeO2 coating for lithium-ion batteries[J]. Electrochimica Acta, 2007, 52: 3236-3241.
[14] WU Chuan, WU Feng, CHEN Li-quan, HUANG Xue-jie. Fabrications and electrochemical properties of fluorine-modified spinel LiMn2O4 for lithium ion batteries[J]. Solid State Ionics, 2002, 152/153: 327-334.
[15] WU Chuan, WANG Zhao-xiang, WU Feng, CHEN Li-quan, HUANG Xue-jie. Spectroscopic studies on cation-doped spinel LiMn2O4 for lithium ion batteries[J]. Solid State Ionics, 2002, 144: 278-285.
[16] BAI Ying, WU Chuan, WU Feng, WANG Guo-qing. Cyclic voltammetry studies on 4 V and 5 V plateaus of non-stoichiometric spinel Li1+xMn2-yO4[J]. Trans Nonferrous Met Soc China, 2006, 16: 402-408.
Foundation item: Project (2002CB211800) supported by the National Basic Research Program of China; project (000Y05-21) supported by the Excellent Young Scholar Research Fund of Beijing Institute of Technology; project (20060542012) supported by the Teaching and Research Fund of Beijing Institute of Technology
Corresponding author: WU Chuan; Tel: +86-10-68912657; E-mail: chuanwu@bit.edu.cn


