J. Cent. South Univ. (2014) 21: 2604-2611
DOI: 10.1007/s11771-014-2219-6
Pre-dispersed carbon black as conductive agent for LiFePO4 cathodes
ZHANG Zhi-an(张治安)1, QU Chang-ming(屈长明)1, JIA Ming(贾明)1, LAI Yan-qing(赖延清)1, 2, LI Jie(李劼)1, 2
1. School of Metallurgy and Environment, Central South University, Changsha 410083, China;
2. Engineering Research Center of Advanced Battery Materials of Ministry of Education, Changsha 410083, China
 Central South University Press and Springer-Verlag Berlin Heidelberg 2014
Central South University Press and Springer-Verlag Berlin Heidelberg 2014
Abstract:
High dispersed carbon black was applied for LiFePO4 cathodes as conductive agent. Nano-conductive carbon agent was pre-dispersed with poly acrylic acid (PAA) as dispersant in organic N-methyl-pyrrolidone (NMP) solvent system. The dispersion property of nano-conductive carbon agent was evaluated using particle size distribution measurements, scanning electron microscopy (SEM) and transmission electron microscope (TEM). LiFePO4 cathode with as-received nano-conductive carbon agent (SP) and LiFePO4 cathode with pre-dispersed nano-conductive carbon agent (SP-PAA) were examined by scanning electron microscopy (SEM), cyclic voltammetry (CV), electrochemical impendence spectroscopy (EIS) and charge/discharge cycling performance. Results show that the dispersion property of carbon black is improved by using PAA as the dispersant. The LiFePO4 cathodes with SP-PAA exhibit improved rate behaviors (4C, 135.1 mAh/g) and cycle performance (95%, 200 cycles) compared to LiFePO4 cathodes with SP (4C, 103.9 mAh/g and 83%, 200 cycles). Because pre-dispersed carbon black (SP-PAA) is dispersed homogeneously in the dried composite electrode to form a more uniform conductive network between the active material particles, electrochemical performances of the LiFePO4 cathodes are improved.
Key words:
LiFePO4 cathode; carbon black; dispersion; poly acrylic acid;
1 Introduction
Lithium ion battery has been considered as one of the most promising candidates for large-scale application in electric vehicle, hybrid electric vehicle and smart grid due to its excellent chemical and thermal stability, good safety, low toxicity and low cost [1-2]. Lithium ion battery electrodes are mainly composed of the active material, conductive agent, binder and solvent.
The conductive agent plays an important role in improving cell performance, especially in regards to cycle life [3-4]. The conductive carbon materials which have good conductivity and electrochemical stability are used as a conductive agent in lithium ion battery, especially nano-conductive carbon additives, such as acetylene black (AB), Ketjen black (KB), Super P (SP) and carbon nanotubes [5-6]. However, it is really difficult to make these kinds of nano-conductive carbon particles disperse homogeneously into the slurry because they have a tendency to flocculate due to their large surface area and high oil adsorption, especially when the particulates must be dispersed in highly dense suspensions of active materials [7-8]. If the conductive particulates are dispersed heterogeneously in the cathode, the performances of the battery might deteriorate and affect production speed, yield and battery safety [9]. Therefore, well-dispersed performances of nano- conductive carbon particles in the cathode material play an important role in battery performance.
Lithium iron phosphate (LiFePO4) has received great attention as a promising alternative cathode material for rechargeable lithium-ion batteries due to its high theoretical capacity of 170 mAh/g, flat voltage at about 3.4 V, and good thermal and chemical stability. Moreover, it offers economic and environmental advantages as a low cost and less toxic material [10]. Nevertheless, the basic LiFePO4 electrode material suffers from low electron conductivity, which limits its capacity and rate capability [11-12]. In order to promote the application of LiFePO4 materials in lithium ion battery, the conductive agent, especially the nano- conductive agent must be added into the slurry of LiFePO4 cathodes. The development of the conductive agent with the high dispersion properties is very important in the production of LiFePO4 cathodes. Researchers tried to use chemical dispersants to improve the dispersion of the conductive carbon and thus improve the performances of the battery. Recently, LEE et al [13] reported the surface-modified carbon black induced by UV/ozone and triethylenetetramine as a dispersant. Modifications to the surface of carbon black result in improved dispersion properties, which in turn enhance the compactness and homogeneity of the microstructure of ink-jet-printed LiCoO2 electrodes. Both LiCoO2 and graphite powders can be dispersed by PAA-NH4 used as a dispersant in aqueous system, resulting in a decrease of powder aggregation and an increase in the contact area between LiCoO2 and the conductive additive [14].
In this work, we choose nano-conductive carbon agent Super P (SP) which is used commonly in commercial lithium ion batteries as conductive additive, and pre-dispersed SP by the dispersant of PAA in NMP solvent (N-methyl-pyrrolidone) that it is the most common industrial organic solvent-based system in battery manufacture. We investigate the physical and chemical properties of the pre-dispersed and conventional SP, respectively, and then investigate their effects on the performance of LiFePO4 cathodes for lithium ion batteries. The dispersion properties of SP were characterized through particle size distribution analysis, scanning electron microscopy and transmission electron microscope. The charge/discharge cycling performance electrochemical redox behavior and impendence of LiFePO4 cathodes formed with pre-dispersed or conventional SP will be examined by using a coin-type cell.
2 Experimental
The commercial carbon-coated LiFePO4 powders with detailed specifications as follows: carbon content of 2%, (mass fraction), average particle size of 1.3 μm, tap density of 1.35 g/cm3 and BET specific surface area of 13.7 m2/g. Super P with an average particle size of 40 nm was acquired from TIMCAL Graphite & Carbon, Switzerland. PAA powder (relative molecular mass Mw=130000) was purchased from Sigma-Aldrich. PVDF (HSV900, Mw=1000000) was supplied by Arkema, France.
Pre-dispersed suspensions of nano-conductive carbon agent were prepared by slowly adding the required amount of dry SP carbon black powder to N-methyl-pyrrolidone (NMP, Merck) with/without a dispersing agent (PAA). These ingredients were mixed (PAA 35% vs SP quantity, solid content at 10% with NMP) by a Polytron PT 10-35 homogenizer at 20000 r/min for 10 min until uniform. The forgoing procedure produces two types of suspensions that SP without PAA and SP with the PAA dispersant. And the two conductive agents with different dispersion properties were named as SP and SP-PAA in the following sections, respectively.
Cathodes were prepared by combining the solid components as SP/SP-PAA (10%, mass fraction), PVDF (10%, HSV 900) and LiFePO4 (80%) in NMP solvent of mass equal to the combined mass of the solid components.
The mixing was performed in a Polytron PT 10-35 homogenizer at 20000 r/min for 20 min until uniform. The mixed slurry was coated onto aluminum foil and dried at 110 °C under vacuum for 24 h to remove the residual solvent.
The electrochemical performances of the LiFePO4 cathodes were evaluated in CR2025-size button LiFePO4/Li half cells, which were assembled by using Celgard 2400 membrane as the separator and filled with 85 μL of liquid electrolyte. The lithium electrode in this half cell was used as the counter electrode as well as the reference electrode and the electrolyte is 1 mol/L LiPF6 dissolved in EC/DMC/EMC 1:1:1 by mass co-solvent, which was purchased from Zhangjiagang Guotai-Huarong New Chemical Materials Co., Ltd., China. All the cells were assembled in an argon-filled glove box (Universal 2440/750, Mikrouna Mech. Tech. Co., Ltd., water content<1×10-6, oxygen content<1×10-6).
2400 membrane as the separator and filled with 85 μL of liquid electrolyte. The lithium electrode in this half cell was used as the counter electrode as well as the reference electrode and the electrolyte is 1 mol/L LiPF6 dissolved in EC/DMC/EMC 1:1:1 by mass co-solvent, which was purchased from Zhangjiagang Guotai-Huarong New Chemical Materials Co., Ltd., China. All the cells were assembled in an argon-filled glove box (Universal 2440/750, Mikrouna Mech. Tech. Co., Ltd., water content<1×10-6, oxygen content<1×10-6).
The cyclic voltammograms (CV) and electrochemical impedance spectra (EIS) of the half cells were measured by using PARSTAT 2273 electrochemical measurement system (PerkinElmer Instrument. USA). CV test was performed at a scan rate of 0.1 mV/s. For the EIS test, the frequency window was between 1 MHz and 0.01 Hz, with amplitude of 5 mV. The obtained EISs were fitted by using ZView software (Scribner and Associates). The charge-discharge performances were tested between 2.5 V and 4.2 V (vs Li/Li+) at different rates on a Land charge/discharge instrument, China. Scanning electron microscopy (SEM, Nova NanoSEM230, operated at 10 kV) and transmission electron microscope (TEM, Tecnai G220 S-Twin) were applied to observe the surface morphology of cathodes and materials. The average apparent particle size of the SP was analyzed by a particle size analyzer. Particle size was measured by Malvern Zetasizer Nano ZS Dynamic Light Scattering (DLS) at 22 °C.
3 Results and discussion
3.1 Dispersions of SP and SP-PAA
Particle size distributions of SP and SP-PAA in the NMP solvent are measured in order to evaluate their agglomeration state, and the results are shown in Fig. 1. We can see that the SP apparent particle size in the NMP solution (D50) is about 3.34 μm, whereas the SP-PAA apparent particle size in the NMP solution (D50) is 0.47 μm. In both cases, the suspended SP particles are quite larger than the initial 40 nm of the as-supplied SP powder, demonstrating a basic flocculation tendency of the NMP-suspended SP particulates because the poor compatibility between hydrophobic carbon black and NMP (polar energy ~12.3 MPa1/2) [13, 15]. But the particle size of SP-PAA is significantly smaller than the SP, besides, the particle size distribution is significantly narrower, which indicates that the particle size is more uniform. Particle size and distribution are affected by the dispersion properties of SP in NMP. High dispersion results in the particle size distributions of SP shifting to the left, i.e., toward smaller particle diameters. These improved dispersion properties of SP-PAA originate from that PAA has a favorable conformation for dispersion of particles due to electrostatic repulsive forces [16].

Fig. 1 Particle size distribution of SP in NMP:
These improved dispersion properties of SP-PAA were also demonstrated by analyzing the SEM micrographs of SP and SP-PAA suspensions, the test slurries were coated on separate Al foils. Figure 2 shows the SEM images of the particles of SP and SP-PAA, respectively. There are no significant differences from the SEM imaging, SP and SP-PAA particles formed aggregate. Comparing Figs. 2 (a) and (b) carefully, we can find that the cavity on the sample surface is reduced evidently in Fig. 2 (b). This suggests that the particles size distribution of SP-PAA becomes more uniform than that of SP. In order to further characterize the dispersion differences of SP and SP-PAA, the microstructures were also studied by TEM.

Fig. 2 SEM micrographs of suspension coated on Al foil prepared at 25 °C:
Figure 3 shows the TEM images of SP and SP-PAA samples, respectively. There is no much difference between the crystal sizes of SP in both samples, about 40 nm, which is consistent with the data provided by the SP manufacturer. The micron-sized particles of the SP sample show large and dense appearance, and reunite with each other to cause the formation of heavy agglomeration. By contrast, the SP-PAA sample grains with only soft contact between each other disperse very well. From Fig. 3, we find that the grain size of the SP-PAA sample decreases obviously, and the phenomenon of heavy agglomeration also reduces. This suggests that adding PAA to SP could improve its dispersion property in NMP.
In order to investigate the stability of SP and SP-PAA suspensions, we tested the sedimentation experiment. Figure 4 shows the sedimentary effect of two kinds of suspensions. It can be seen that the SP-PAA apparent particle size and suspension behavior in NMP shows no significant change after aging 15 d under static storage at room temperature, which indicates that the SP-PAA suspension is stable. In distinct contrast, the same batch of SP which without PAA dispersant, gradually sank, collected at the bottom of the NMP solution, which indicates that the SP cannot suspense in the NMP solution stable for a long time. The stability of colloidal suspensions can be greatly improved by using a dispersant. The better the nano-particles’dispersion, the more stable the suspensions [17-18]. Therefore, it suggests that SP-PAA particulates have better dispersion property and the suspension is stable. The result is consistent with the particle size distribution analysis. It is thus seen that PAA is effective for preventing or minimizing aggregation of nano-scale SP particles produced from powder aggregates in NMP solution.
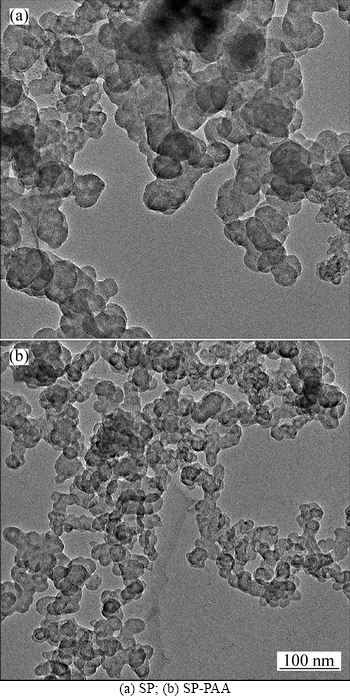
Fig. 3 TEM images of SP suspension:
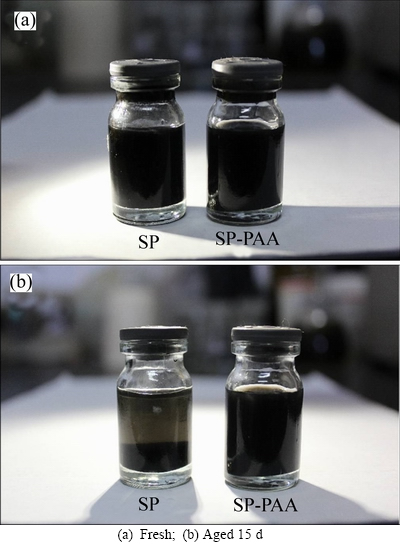
Fig. 4 Sedimentation effect of SP suspension:
3.2 Performance of LiFePO4/SP and LiFePO4/SP-PAA cathodes
The cyclic voltammograms of the LiFePO4/SP and LiFePO4/SP-PAA cathodes are shown in Fig. 5, respectively. A pair of redox peaks appear in the CV curves, which are typical redox peaks of LiFePO4 electrode [19]. It is clear that LiFePO4/SP-PAA cathode, the oxidation peaks shift to the left, the reduction peaks shift to the right, the voltage difference between the redox peaks decreases from 0.341 V to 0.218 V, and the redox peaks are much sharper than those of the LiFePO4/SP cathode. These confirm that the redox reaction rate of the LiFePO4/SP-PAA cathode is faster, which indicates the weakening of polarization in the battery, better kinetics characteristics and better reversibility of LiFePO4/ SP-PAA cathode. Since both cathodes are fabricated identically except for the inclusion of PAA, this confirms that using PAA during cathode fabrication improves the dispersion of the SP, presumably because the electron transfer rate increases by more uniform conductive channels. The curves of sweeps 1 and 2 for the LiFePO4/ SP-PAA cathodes are very similar in shape. It should be noted that the dispersant PAA is electrochemically stable over the 2.5-4.5 V voltage range.
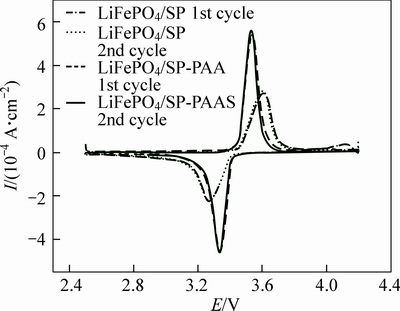
Fig. 5 Cyclic voltammograms of LiFePO4/SP and LiFePO4/ SP-PAA cathodes (Sweep rate is 0.1 mV/s)
The 3rd charge and discharge curves of the LiFePO4/SP and LiFePO4/SP-PAA cathodes between 2.5 V and 4.2 V voltage limits at 0.1C and 4C are shown in Fig. 6. Here, we choose the curves of the 3rd cycle for the reason that the charge and discharge curves before it may not be quite stable. It is clear that the discharge capacities for LiFePO4/SP and LiFePO4/SP-PAA cathodes are 153.2 and 165 mAh/g at 0.1C, but when the discharge current is 4C, the discharge capacities are 103.9 and 135.1 mAh/g, respectively. Compared with the LiFePO4/SP cathode, LiFePO4/SP-PAA cathode has a better specific capacity, especially in the case of high rate. The phenomena are in accordance with the report from Ref. [13]. Moreover, the LiFePO4/SP-PAA cathode shows a lower charge plateau potential and a higher discharge plateau potential than the LiFePO4/SP cathode and the plateau potential differences are 0.321 V and 0.463 V at 4C, respectively. The potential drop between the charge and discharge plateaus became lower that indicating better kinetics characteristics and better reversibility of the LiFePO4/SP-PAA cathode, which can be ascribed to the lower potential polarization of the cathode during the charge and discharge processes. This trend is consistent with the dispersion properties of SP. The improved specific discharge capacities of LiFePO4/SP-PAA cathode can be attributed to the SP which is more homogeneously dispersed, improving the electrical contact between the SP and LiFePO4 nano-particles and can obtain the lower potential polarization of the cathode during the charge and discharge processes.
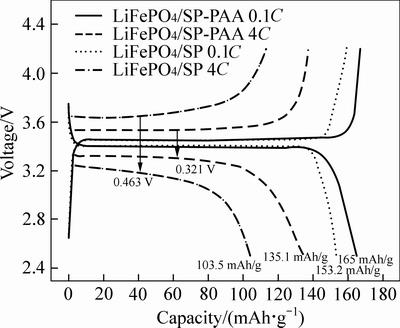
Fig. 6 Discharge and charge curves of LiFePO4/SP and LiFePO4/SP-PAA cathodes at 0.1C and 4C (1C=170 mA/g)
The rate behaviors of the prepared LiFePO4/SP and LiFePO4/SP-PAA cathodes were evaluated and the results are shown in Fig. 7. It is found that the specific discharge capacity of the LiFePO4/SP-PAA cathode is not much different from that of the LiFePO4/SP cathode at 0.1C, the gap is 11 mAh/g. However, with the increase of the discharge current, the gap becomes larger, especially at 4C, and the LiFePO4/SP-PAA cathode shows a specific discharge capacity, the specific discharge capacity of the LiFePO4/ SP-PAA cathode is 31 mAh/g more than that of the LiFePO4/SP cathode. That indicates that the LiFePO4/SP-PAA cathode has a better specific discharge capacity exhibition than the LiFePO4/ SP cathode at different discharge rates. The result shows that the LiFePO4/SP-PAA cathode has better rate capability.

Fig. 7 Cycling behavior of LiFePO4/SP and LiFePO4/SP-PAA cathodes at different charge/discharge rates
Figure 8 presents cycle performances of LiFePO4/ SP-PAA cathode and LiFePO4/SP at 1C between 2.5 V and 4.2 V. Comparing the two curves, it shows that the specific discharge capacity and cycle performance of LiFePO4/SP-PAA cathode are better than thoes of LiFePO4/SP cathode. With the increase of the cycle number, the gap becomes larger. By 200 cycles, the discharge capacity of the LiFePO4/SP cathode is 139.7 mAh/g for the initial cycle and decreases to 116.4 mAh/g, the capacity retention ratio is only 83%. Whereas by 200 cycles, the discharge capacity of LiFePO4/SP-PAA cathode is 150.8 mAh/g for the initial cycle and a high reversible capacity of 142.2 mAh/g is retained with the capacity retention ratio of 95%. These results confirm that the LiFePO4/SP-PAA cathode significantly enhances cell cycle performance.
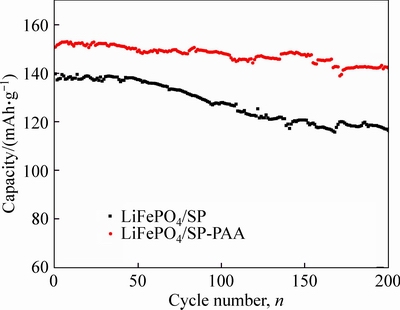
Fig. 8 Cycle performances of LiFePO4/SP cathode and LiFePO4/SP-PAA cathode at 1C between 2.5 V and 4.2 V
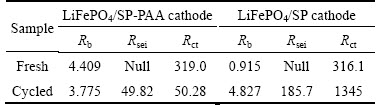
In order to understand more detail about the effect of SP-PAA, EIS method was used for measuring the cells containing the LiFePO4/SP-PAA cathode and the LiFePO4/SP cathode respectively, which can help to clarify the ohmic resistance and polarization resistance behavior coincident with capacity loss. Figure 9 shows Nyquist plots obtained from the LiFePO4/SP-PAA and the LiFePO4/SP cells which were just assembled and completed the cycle test. The impedance of the cells after cycle test are measured at a fully charged state and analyzed by Zview software. An intercept at the Zreal axis in high frequency corresponded to the ohmic resistance (Rb), which represents the total resistance of the electrolyte, separator, and electrical contacts. The depressed semicircle in the high frequency range is related to the Li-ion migration resistance (Rsei) through the SEI film formed on the electrode or another coating layer. Second semicircle in the middle frequency range indicates the charge transfer resistance (Rct). The inclined line in the lower frequency represents the Warburg impedance, which is associated with lithium-ion diffusion in the LiFePO4 particles [20-22]. All the spectra were well fitted by an equivalent circuit model in Fig. 9, in which Rb stands for the resistance of cell bulk including the electrolyte, electrode and separator, Rsei and Csei mean resistance and capacitance of the SEI, respectively. Rct is the charge-transfer resistance, Cdl acts as the double-layer capacitance, and W is the Warburg resistance [23]. Cell resistance (Rb, Rsei and Rct) values are provided in Table 1. Because the SEI film has not formed on the electrode when the cells are just assembled, the LiFePO4/SP and LiFePO4/SP-PAA fresh cathodes do not have Rsei. It shows that the values of Rb and Rct of the LiFePO4/SP-PAA cathode are reduced compared to the LiFePO4/SP cathode when they complete the cycle test, explaining the better rate capability and cycle performance in Fig. 7 and Fig. 8, respectively. In particular, the charge transfer resistance Rct of the LiFePO4/SP cathode increases drastically from 316.1 Ω to 1345 Ω after cycle test, while it is interesting to find that the Rct of the LiFePO4/SP-PAA cathode is decreased from 319 Ω to 50.28 Ω after cycle test. Here the difference in resistance of the cell may be caused by the better dispersion property of SP-PAA and more uniform distribution in the cathode. It could maintain effective contact between the actives even after 200 cycles. The electron transfer rate is increased by more uniform conductive channels, thereby improving the electrochemical performance of the battery.
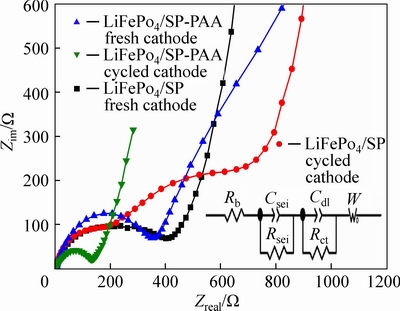
Fig. 9 Nyquist plots of LiFePO4/Li half cells containing LiFePO4/SP-PAA and LiFePO4/SP cathodes
Table 1 Impedance parameters calculated from equivalent circuits (Ω)
The effect of additions of SP and SP-PAA on the dispersion homogeneity of cathode powders is also assessed by analyzing the SEM micrographs of electrode sheets. Figures 10(a) and (c) show microstructures of the top surfaces of LiFePO4 electrode sheets prepared with SP and SP-PAA, respectively. It is obvious that SP forms heavy agglomeration itself, which cannot be well scattered on the LiFePO4 surface to form an effective contact in Fig. 10(a). In contrast, Fig. 10(c) shows that the LiFePO4 cathode prepared with SP-PAA possesses better uniformity and smaller particle size of SP-PAA cover on the LiFePO4 particles which suggests an improved electrical contact between the SP-PAA and LiFePO4 particles. Compared to the cathode with SP, the particles of the cathode with SP-PAA could form a better conductive network that reduces the polarization. So, the rate capability of the battery could be enhanced.
Figures 10(b) and (d) show the SEM images of the battery film after 200 cycles. The images show that the electrode slice surface is coated with a layer of organic film. The particles are wrapped to form a large piece of reunion, which suggests that the contact resistance between active material and conductive agent can increase, thus leading to capacity fading. But in Fig. 10 (d), the morphology of the cathode prepared with SP-PAA is significantly better than the cathode with SP, and the conductive agent is still distributed around the LiFePO4 particles. Therefore, the cathode with SP-PAA showed better cycling performance.

Fig. 10 SEM images:
4 Conclusions
1) Nano-conductive carbon agent can be pre- dispersed with PAA as dispersant in organic N-methyl-pyrrolidone (NMP) solvent system and the dispersion properties of nano-conductive carbon are observed by particle size distribution measurements, SEM and TEM. Results show that the particle size of SP-PAA is significantly smaller than that of the SP, besides, the particle size distribution is significantly narrower, which indicates that the particle size is more uniform and the dispersion properties of SP-PAA are improved.
2) The dispersion properties of nano-conductive carbon agent have a great impact on the electrochemical performance of LiFePO4 cathodes. The LiFePO4 cathodes with SP-PAA exhibit improved rate behaviors and cycle performance compared to LiFePO4 cathodes with SP. At 4C, the discharge capacity of the LiFePO4/SP-PAA cathode still remains 135.1 mAh/g, while the capacity of the LiFePO4/SP cathode is only 103.9 mAh/g. More than 17% of the capacity loss occurs in the LiFePO4/SP cathode at 1C after 200 cycles, while the LiFePO4/SP-PAA cathode loses only 5% after 200 cycles.
3) It is believed that such methodology will have certain advantage for the development of the lithium ion battery technology in the future. Our future work will continue to explore alternative formulae for PAA, as well as various combinations of alternative SP materials.
References
[1] WANG, Yong-gang, PING He, ZHOU Hao-shen. Olivine LiFePO4: development and future [J]. Energy & Environmental Science, 2011, 4(3): 805-817.
[2] LIU Yan-yi, LIU Da-wei, ZHANG Qi-feng, YU Dan-mei, LIU Jun, CAO Guo-zhong. Lithium iron phosphate/carbon nanocomposite film cathodes for high energy lithium ion batteries [J]. Electrochimica Acta, 2011, 56(5): 2559-2565.
[3] WANG Ke, WU Yang, LUO Shu, HE Jia-ping, JIANG Kai-li, FAN Shou-shan. Hybrid super-aligned carbon nanotube/carbon black conductive networks: A strategy to improve both electrical conductivity and capacity for lithium ion batteries [J]. Journal of Power Sources, 2013, 233: 209-215.
[4] DOMINKO R, GABERSCEK M, DROFENIK J, BELE M, PREJOVNIK S, JAMNIK J. The role of carbon black distribution in cathodes for Li ion batteries [J]. Journal of Power Sources, 2003, 119: 770-773
[5] VARZI A, TAUBERT C, WOHLFART M, KREIS M, SCHUIZ W. Study of multi-walled carbon nanotubes for lithium-ion battery electrodes [J]. Journal of Power Sources, 2011, 196(6): 3303-3309.
[6] SPAHR M E, GOERS D, LEONE A, STALLONE S, GRIVEI E. Development of carbon conductive additives for advanced lithium ion batteries [J]. Journal of Power Sources, 2011, 196(7): 3404-3413.
[7] LIU Zhao-lin, LEE J Y, LINDNER H J. Effects of conducting carbon on the electrochemical performance of LiCoO2 and LiMn2O4 cathodes [J]. Journal of Power Sources, 2001, 97: 361-365.
[8] WU Bin, BAI Lu, GONG Qian-ming, LIANG Ji. Effect of non-ionic surfactants on the dispersion of multiwalled carbon nanotubes at high loading in ethanol [J]. Acta Phys. Chim. Sin., 2009, 25(06): 1065-1069. (in Chinese)
[9] KURODA S, TOBORI N, SAKURABA M, SATO. Charge–discharge properties of a cathode prepared with ketjen black as the electro- conductive additive in lithium ion batteries [J]. Journal of Power Sources, 2003, 119: 924-928.
[10] CHEN Zhao-hui, DAHN J. R. Reducing carbon in LiFePO4/C composite electrodes to maximize specific energy, volumetric energy, and tap density [J]. Journal of the Electrochemical Society 2002, 149(9): A1184-A1189.
[11] PROSINI P P, LISI M, SCACCIA S, CAREWSKA M, CAROELLNI F, PASQULAI M. Synthesis and characterization of amorphous hydrated FePO4 and its electrode performance in lithium batteries [J]. Journal of the Electrochemical Society, 2002, 149(3): A297-A301.
[12] WANG G X, YANG L, BEWLAY S L, CHEN Y, LIU H K, AHN J H. Electrochemical properties of carbon coated LiFePO4 cathode materials [J]. Journal of Power Sources, 2005, 146(1): 521-524.
[13] LEE J H, WEE S B, KWON M S, KIM H H, CHOI J M, SONG M S, PARK H B, KIM H, PAIK U. Strategic dispersion of carbon black and its application to ink-jet-printed lithium cobalt oxide electrodes for lithium ion batteries [J]. Journal of Power Sources, 2011, 196(15): 6449-6455.
[14] LI C C, LEE J T, PENG X W. Improvements of dispersion homogeneity and cell performance of aqueous-processed LiCoO2 cathodes by using dispersant of PAA–NH4 [J]. Journal of the Electrochemical Society, 2006, 153(5): A809-A815.
[15] HANSEN C M. Hansen solubility parameters: A user’s handbook [M]. Boca Raton: CRC PressI Llc, 2007: 200-201.
[16] LEE J H, PAIK U, HACKLEY V A, CHOI Y M. Effect of poly (acrylic acid) on adhesion strength and electrochemical performance of natural graphite negative electrode for lithium-ion batteries [J]. Journal of Power Sources, 2006, 161(1): 612-616.
[17] SONG Y L, LIU X L, ZHANG J Q, ZOU Xin-yang, CHEN Jian-feng. Rheological properties of nanosized barium titanate prepared by HGRP for aqueous tape casting [J]. Powder technology, 2005, 155(1): 26-32.
[18] ZHAO Wei, YANG De-an, YIN Xiu-xin, XU Tian-xian. Study on stability of Nano-Al2O3 aqueous suspension [J]. Key Engineering Materials, 2004, 280: 1023-1026.
[19] LUO Guo en, YUShi xi, SHAO Dan, XIAO Jian cai, YU Xiao yuan. Synthesis and characterization of core shell nano LiFePO4/C cathode material [J] Journal of Central South University of Technology, 2011, 42(7): 1942-1946. (in Chinese)
[20] SUN A, ZHENG J, SHENG Q. A highly sensitive non-enzymatic glucose sensor based on nickel and multi-walled carbon nanotubes nanohybrid films fabricated by one-step co-electrodeposition in ionic liquids [J]. Electrochimica Acta, 2012, 65: 64-69.
[21] LIU Yun-jian, LI Xin-hai, GUO Hua-jun, WANG Zhi-xing, PENG Wen-jie, YANG Yong, LIANG Ru-fu. Effect of carbon nanotube on the electrochemical performance of C-LiFePO4/graphite battery [J]. Journal of Power Sources, 2008, 184(2): 522-526.
[22] MOSS P L, AU G, PLICHTA E J, ZHENG J P. Investigation of solid electrolyte interfacial layer development during continuous cycling using AC impedance spectra and micro-structural analysis [J]. Journal of Power Sources, 2009, 189(1): 66-71.
[23] SHIN H C, CHO W I, JANG H. Electrochemical properties of the carbon-coated LiFePO4 as a cathode material for lithium-ion secondary batteries [J]. Journal of Power Sources, 2006, 159(2): 1383-1388.
(Edited by DENG Lü-xiang)
Foundation item: Project(51204211) supported by the National Natural Science Foundation of China; Project(2012M521543) supported by the China Postdoctoral Science Foundation
Received date: 2013-01-31; Accepted date: 2013-06-04
Corresponding author: JIA Ming, PhD; Tel: +86-731-88830649; E-mail: jiamingsunmoon@yahoo.com.cn
Abstract: High dispersed carbon black was applied for LiFePO4 cathodes as conductive agent. Nano-conductive carbon agent was pre-dispersed with poly acrylic acid (PAA) as dispersant in organic N-methyl-pyrrolidone (NMP) solvent system. The dispersion property of nano-conductive carbon agent was evaluated using particle size distribution measurements, scanning electron microscopy (SEM) and transmission electron microscope (TEM). LiFePO4 cathode with as-received nano-conductive carbon agent (SP) and LiFePO4 cathode with pre-dispersed nano-conductive carbon agent (SP-PAA) were examined by scanning electron microscopy (SEM), cyclic voltammetry (CV), electrochemical impendence spectroscopy (EIS) and charge/discharge cycling performance. Results show that the dispersion property of carbon black is improved by using PAA as the dispersant. The LiFePO4 cathodes with SP-PAA exhibit improved rate behaviors (4C, 135.1 mAh/g) and cycle performance (95%, 200 cycles) compared to LiFePO4 cathodes with SP (4C, 103.9 mAh/g and 83%, 200 cycles). Because pre-dispersed carbon black (SP-PAA) is dispersed homogeneously in the dried composite electrode to form a more uniform conductive network between the active material particles, electrochemical performances of the LiFePO4 cathodes are improved.


