
Preparation and performance of fluorescent sensing coating for monitoring corrosion of Al alloy 2024
LI Song-mei(李松梅), ZHANG Hong-rui(张洪瑞), LIU Jian-hua(刘建华)
School of Materials Science and Engineering, Beijing University of Aeronautics and Astronautics,
Beijing 100083, China
Received 10 April 2006; accepted 25 April 2006
Abstract:
A kind of fluorescent sensing coating was prepared for monitoring corrosion of aluminum alloys by incorporating phenylfluorone(PF) into acrylic paint as sensing material. The fluorescent dye PF reacts with aluminum ions on corroded aluminum substrate to occur fluorescence quenching observed in UV light. This paint system is sensitive to underlying corrosion processes through reacting with the Al3+ produced by anodic reaction accompanying corrosion. After a certain time, when the samples of Al alloy 2024 coated with PF-acrylic paint were immersed in 1 mol/L NaCl solution, fluorescence quenching spots can be seen with unaided eyes. With the development of corrosion process, the size of fluorescence quenching spots increases. Active corrosion areas on the sample surface were found under the fluorescence quenching spots by optical microscope. The corrosion areas can be observed more clearly by SEM, and many pits are found. This suggests that the fluorescence quenching spots are the sites of produced Al3+ by the anodic reaction of the local attack of the coated Al alloy substrate in the chloride solution and the corrosion process of the coated Al alloy can be monitored on-line by the sensing coating. The sensitivity of this coating system for detection of anodic reaction associated with corrosion was determined by applying constant charge current and measuring the charge, at which fluorescence quenching is detected in the coating with unaided eyes. Visual observation of coated samples can detect fluorescence change resulting from a charge corresponding to an equivalent hemispherical pit with approximate depth of 50 μm.
Key words:
aluminum alloy 2024; fluorescence; sensing coating; corrosion monitoring; sensibility;
1 Introduction
Aluminum alloys have been widely used in manufacture of airplane and watercraft because of perfect mechanical and machinable performance. However, structural aluminum alloys are corroded in the sea environment that the invalidation and destroy of structural aluminum alloy due to localized corrosion is one of primary forms that make airplane and watercraft invalidate[1]. Traditional methods for corrosion detection are not applicable since corrosion may occur in relatively inaccessible locations, such as deep within the lap joints of the skin of an airplane. Many different sensors and techniques are being developed to detect corrosion[2-5], for example, electrochemical sensors, electrical resistance sensors, passive sensors and optical fiber sensors. These methods are limited in their ability to monitor the corrosion process and its progression in-situ, visualize the corroded sites and reflect the characters of the corrosive conditions, which are at or proximal to the metal surface.
Other sensors are developed in order to identify the locations of the corroded sites in-situ and monitor the response with the corrosion process, such as array sensor[6] and neutron radiography[7], and so on. Some fluorescent compounds and color–change dyes are so sensitive to underlying corrosion processed by reacting the corrosion product or the pH change associated with the anodic or cathodic reaction, that fluorescence based chemical sensors gained much attention for early detection of corrosion. Fluorescing and color-change compounds were applied to Al after corrosion to identify the locations of the hydrous aluminum oxide corrosion product in the early 1980s. Smyrl investigated corrosion initiation on Al2024-T3 via fluorescence probe and characterized the initial state of pitting in situ[8]. Sibi used SABF, lumogallion and Phen Green to study the selective corrosion process of Al-Cu-Mg phase under the epoxy coating[9]. SZUNERITS et al[10] researched Al surface corrosion in Al-Cu galvanic couple cell with the SNAFL and morin. JOHNSON et al[11] and AGARWALA et al[12] identified possible fluorescent compounds for corrosion detection in coatings, and they used some of them to prepare smart coatings. ZHANG et al[13] discussed the sensitivity of acrylic base- fluorescent dye coating by electrochemical method.
In our laboratory, fluorescent materials were being incorporated into paint coatings for detecting corrosion. Fluorescent compounds are added into the coatings in the form of pigment, which has some advantages, the fluorescent compounds will not reduce the stability and corrosion defend ability of coatings, and the corrosion sensing coating can monitor all the coated metal surface. In order to make the fluorescent dyes have good soluble performance, dispersive performance and high sensitivity in the coating, different surfactants are added at the same time. In this work, acrylic base-fluorescence coating is prepared using phenylfluorone(PF) as fluorescent sensing material which is sensitive to the aluminium ion and Triton X-100 as surfactant which can increase PF’s stability and sensitivity. The fluorescence change of the sensing coating is observed by unaided eyes with a hand-held UV light. The availability of the coating system is approved by observing metal substrate under the fluorescent site with optical microscope and SEM. The sensitivity of this coating system for detecting anodic reaction associated with corrosion was determined by applying constant charge current and measuring the charges, at which the fluorescence quenching is detected.
2 Experimental
2.1 Materials
The fluorescent compound phenylfluorone and surfactant Triton X-100 were purchased from C.P. Research Chemicals Ltd(US) and were not purified any further. Metal samples (50 mm×30 mm×1 mm) were cut from an Al2024-T3 sheet(nominal composition: 3.8%-4.9% Cu, 1.2%-1.8% Mg, 0.3%-0.9% Mn, 0.3%Fe, 0.2%Si, 0.1%Zn, 0.1%Ti, balance Al). Before testing, the samples were mechanically polished to 800 grit SiC paper, and then cleaned ultrasonically with ethyl alcohol. Clear acrylic paint(88B01,Areonautics material research institute, Beijing) were mixed with 0.5% (mass fraction) PF and 1.0%(mass fraction) Triton X-100. The indicating layers were coated on the surface of Al alloy samples. After 8 h, these samples were then top-coated with a uniformly-sprayed clear acrylic (containing no indicating compounds and surfactant). The combined thickness of the two layers was (30![]() 5) μm. The samples were corroded in 1mol/L sodium chloride solution. A hand-held UV light was prepared to observe the fluorescent coatings with unaided eyes.
5) μm. The samples were corroded in 1mol/L sodium chloride solution. A hand-held UV light was prepared to observe the fluorescent coatings with unaided eyes.
2.2 Fluorescent measurement
Florescent measurements were taken with fluorescent spectrophotometer RF-5401. A series of standard aluminium ion solutions of different concentration were added to the PF solution, in which the fluorescence quenching degree was detected.
2.3 Microscopic observation
The metal surface was observed by OLYMPUS XJZ-1A microscope without removing the coating after fluorescence quenching spots could be found with UV light. Scanning electron microscopy(SEM) was taken to examine the local pits under the fluorescence quenching spot.
2.4 Sensitivity measurement
The sensitivity of this coating system was determined by applying constant anodic current and measuring the charge, at which fluorescence quenching spot was detected. Sensitivity measurement was carried out using Princeton263A analyzer. Constant anodic current densities from 100 nA/cm2-50 μA/cm2 were applied to test samples immersed in 1 mol/L sodium chloride solution, using a Pt counter as electrode. The samples were immersed in the solution for 30 min before each test. The test was stopped when the first fluorescence quenching spot was observed.
3 Results and discussion3.1 Characteristics of sensing material
Phenylfluorone is an important color reagent. A great development has been made using phenylfluorone to detect trace aluminium ion[14,15]. Typical absorption spectrum of PF and PF-Al3+ compound is illustrated in Fig.1. Pure PF has an absorption peak at 483 nm, while the absorption peak of PF-Al3+ changes to 510nm. The formation of the coordination compounds PF-Al3+ results in fluorescence quenching. The trend of fluorescence quenching depended on the aluminium ion concentration is shown in Fig.2. It is obviously found that the fluorescence intensity of PF is greatly quenched by the aluminium ion, which can be also observed by eyes. Fluorescence of PF is also affected by pH and temperature, because pH change involves gain or loss of ionizable hydrogen atoms, which changes the conjugation of the molecule and affects the fluorescence. Some researchers reported that the optimal pH for phenylfluorone is 9-10, which is the very corrosion condition.
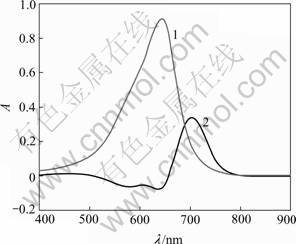
Fig.1 Absorption spectra of PF(1) and PF-Al3+ (2)
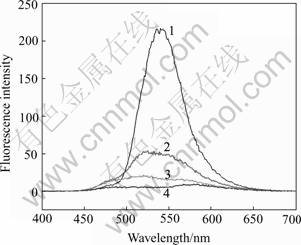
Fig.2 Fluorescence of PF without Al3+(1) and with 10% Al3+(2), 20% Al3+(3) and 30%Al3+(4)
It is well known that PF is sensitive to aluminium ion, but the sensitivity of PF may change when this compound is mixed with an organic matrix and applied to a metal surface. In order to determine the critical aluminium ion concentration, the sensing coating could response, a simulative corrosion test was performed on samples consisting of glass slides coated with the indicating layer, but no top coat. These samples were immersed in deionized water and AlCl3 was slowly added to monitor the fluorescence until a fluorescence quenching spot was observed. The sensing coatings contain different concentrations of PF from 0.1% to 0.5%. The results are shown in Table 1. If the response time is longer than 1 Ms, we consider the coating can not detect the aluminium ion on-line. The results show that the coating containing 0.5% PF is most sensitive to aluminium ion, and the critical concentration for this coating is 10 μg/mL.
Table 1 Critical aluminium ion concentration of different sensing coatings containing different PF

3.2 Validity of sensing coating
During exposure electrolyte solution concentration, a coating over a metal substrate appears to undergo both physical aging and chemical degradation. As exposure continues, the coating layer damaged above the coating/metal interface accumulates until there begins to be a permanent accumulation of electrolyte at this interface. When chloride ions are present, migration of Cl- into the metal surface may lower the resistance to outward migration of Al3+ and initiating pitting. At the propagation stage, aluminium is dissolved to Al3+ within the pit and pH at the coating/metal, interface will increases. The anodic and cathodic reactions are as follows.
1) Anode:
Al→Al3++3e
2) Cathode:
O2+H2O+4e→4OH-
The sensitivity of the fluorescence coatings has been discussed above. After a certain immersion time, fluorescence quenching spots due to the product Al3+ associated with the anodic reaction appears (Fig.3(a)). The spots are not the actual size of the corroded sites, but the relative locations are shown. The size and fluorescence quenching degree increased with the increase of the immersion time. Fig.3(b) shows photograph of metal surface under the fluorescence quenching spots without Fig.3(b) removing coatings by optical microscope. From Fig.3(b), the corroded area and the movement of bubbles can be obviously observed. MORCILLO considers that the center of the bubbles is the cathodic reaction site. It suggests that the fluorescence quenching spots are, in fact, the corrosion sites of the local attract of coated Al alloy in the chloride solution.
The coatings were removed when fluorescence quenching was serious and the metal surface was observed by SEM. Fig.4(a) shows the fluorescence quenched almost completely and the color of coating changed to clear under UV light. From Fig.4(a), the fluorescence quenching spots display yellow, which is the color of PF-Al3+ compound. A circular corroded pit can be observed from Fig.4(b), the shape of fluorescence
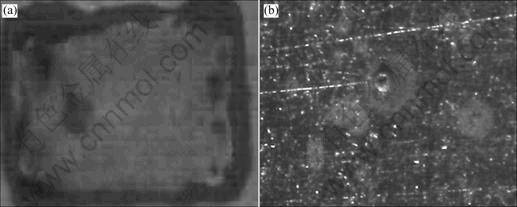
Fig.3 Photographs of fluorescence quenching under UV light(a) and metal surface under fluorescence quenching spots under optical microscope(b)
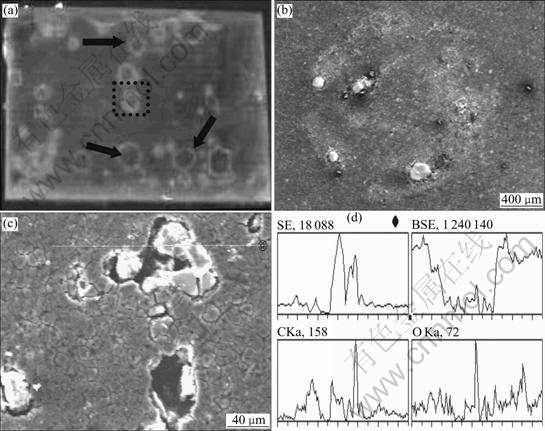
Fig.4 Photographs of fluorescence coating and metal surface analysis: (a) Photograph of fluorescence coating; (b) SEM photograph under fluorescence quenching spot; (c) SEM photograph of corroded site; (d) EDS for Fig.4(c)
quenching spot and deposit of corrosion product can be also found clearly. EDS was taken for the pit, and element carbon was found to be much more in pit than around it (Figs.4(c) and (d)). This proves that the deposit of corrosion product is PF-Al3+compounds. A number of organic compounds were described as aluminum corrosion inhibitors, the majority is nitrogen or oxygen-containing compounds that seem to be absorbed on the aluminum anode and slow down the corrosion rate and prevent adsorption of chloride ion.
3.3 Sensitivity of coating
The sensitivity of the fluorescence coating depends on the response to pH value, corrosion product and other conditions. The character of fluorescence materials will change when they are added to organic paints, then the sensitivity of the fluorescence coating includes two aspects that are the sensitivity to corrosion product, and the sensitivity to the corrosion degree.
The first aspect has been discussed in 3.1 section. In order to determine the sensitivity of the corrosion degree, Frankel method was improved and the constant anodic current densities from 100 nA/cm2-50 μA/cm2 were applied. Since current density, J, is defined as
![]() (1)
(1)
where q is charge density; t is time. The following equation should exit relationship between the elapsed time and current density, until detection.
lgtdet=lgqdet-lgJdet (2)
where tdet is the time at detection; qdet is charge density passed; Jdet is the applied current density. Therefore, a plot of lgtdet vs lgJdet has a slope of -1, and an intercept provides a value of detectable charge density, which is a measure of the sensitivity. The data for PF-acrylic coating fitting to Eqn.(5) are shown in Fig.5.
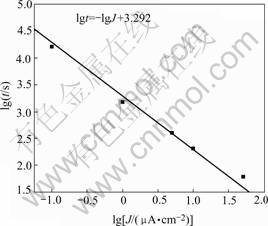
Fig.5 Plot of detection time of fluorescence quenching vs applied anodic current density of Al coated with PF(0.5%)- acrylic coating in 1 mol/L NaCl solution
The detectable charge density is calculated to be 1.95×10-3 C/cm2. Assuming that this amount of charge was generated from a single hemispherical pit, the radius (or depth), r, can be gotten from Faraday law.
![]() (3)
(3)
where s is the sample area; M is the effective atomic mass of Al; ρ is the density of alloy; n is the charge on the dissolved metallic ion; F is Faraday constant.
The applied constant anodic current density does not flow uniformly to the sample surface, but will instead tend to flow to the defective areas in the coating and metal substrate. The current flowing to a given coated sample is distributed among defective points(N). The charge passed at the point of detection is the a total charge Itot, which is equal to N×Idet, where Idet is the critical charge required for detection of each single spot. The previous analysis can thus be modified.
![]() (4)
(4)
![]() (5)
(5)
where Ifix is applied charge; N is the number of defect points. According to this analysis, a plot of lg(tdet/N) vs lgIfix should have a slope of -1, and an intercept that provides a value of detectable charge. Fig.6 shows the data replotted in this fashion. Qdet can be determined to be 7.76×10-3 C. The effective radium is
![]()
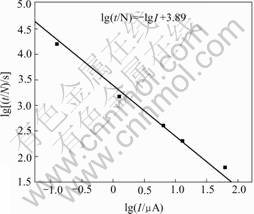
Fig.6 Sensitivity test results of PF(0.5%)-acrylic coating in 1 mol/L NaCl solution
In fact, there was not only one pit on the metal surface after anodic current passed, but many too small and big pits were not all round in shape. Fig.7 shows the pits distribution under a fluorescence quenching spot when 5 μA/cm2 constant anodic current density was applied. It is obvious that the number of pits is not easily counted and the sizes of pits are not the same. Some researchers have indicated that the pits distribution of Al 2024 alloy can be described by Gumbel distribution or Gaussian distribution. So, the critical pit depth deter-
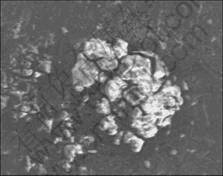
Fig.7 Pits distribution of Al 2024 alloy at 5 μA/cm2 constant anodic current density
mined in this fashion was a nominal depth, but not the real depth. However, the nominal depth used in this study is a measure of the sensitivity of the indicative coating systems. A small critical pit depth is thus associated with a highly sensitive coating.
4 Conclusions
The fluorescence quenching with the Al3+ caused by the anodic reaction in the corrosion process is easily seen with the unaided eye under a hand-held UV light. The sensitivity of these coating systems is determined by passing anodic current and the charge at a fluorescence quenching spot is detected. For this work, an effective pit with size on the order of 50 μm is detectable.
References[1] ZHU Xiang-rong, WANG Xiang-run. Corrosion and Detection for Metals in Sea Environment [M]. Beijing: National Defence Industrial Press, 1999.
[2] GONZALEZ J A, OTERO E, BAUTISTA A. Use of electrochemical impedance spectroscopy for studying corrosion at overlapped joints [J]. Progress in Organic Coatings, 1998, 33: 61-67.
[3] DEKEYSER J C, DE SCHATTER F, VAN DER POORTER C. An electrochemical sodium sensor for aluminium melts [J]. Sensors and Actuators B: Chemical, 1995, 24-25: 273-275.
[4] NAZAROV A, THIERRY D. Rate-determining reactions of atmospheric corrosion [J]. Electrochimica Acta, 2004, 49: 2717-2724.
[5] BENOUNIS M, RENAULT N. Elaboration of an optical fibre corrosion sensor for aircraft applications [J]. Sensors and Actuators B: Chemical, 2004, 100: 1-8.
[6] YANG L, SRIDHAR N, PENSADO Q, DUN D S. An In-Situ Galvanically Coupled Multielectrode Array Sensor for Localized Corrosion [M]. Houston: NACE International TX, 2002.
[7] VERGINIA T, G.JESSE J, SILVA D. Detection of corrosion in aircraft aluminum alloys [J]. App Radiat Isat, 1998, 49(9): 779-782.
[8] BUCHLER M, KERIMO J, GUILLAUME F, SMYRL H W. Investigation of the initiation of localized corrosion on aluminum alloys by using fluorescence microscopy [J]. J Electrochem Soc, 2000, 42(9): 1661-1669.
[9] SIBI M P, ZONG Zhen-gang. determination of corrosion on aluminum alloy under protective coating using fluorescent probes [J]. Progress in Organic Coatings, 2003, 47: 8-15.
[10] SZUNERITS S, WALT D R. Aluminum surface corrosion and the mechanism of inhibitors using ph and metal ion selective imaging fiber bundles [J]. Anal Chem, 2002, 74: 886-894.
[11] JOHNSON R E, AGARWALA V S. Uing fluorescent compounds as early warning detectors for corrosion [J]. Corrosion, 1994, 16: 25-29.
[12] JOHNSON R E, AGARWALA V S. Fluorescence Based Chemical Sensors for Cor-rosion Detection [M]. Houston: NACE International TX, 1997.
[13] ZHANG J, FRANKEL G S. Paint as a corrosion sensor: Acrylic coating systems[J]. Mat Res Soc, 1998, 503: 15-24.
[14] ZHOU Fa-lian, GAO Yan-ming. Spectrophotometric determination of iron, aluminum and copper in 2,6,7-Trihydro×y-9-phenylfluorone- triton x-100 phase-separation system by principal component regression method [J]. Chinese Journal of Analytical Chemistry, 1995, 23(4): 423-425.(in Chinese)
[15] WANG Ping, HUANG Ying-ping. Study on the color reaction of the reagent 4-azo-chromotropic acid-phenylfluorone with alu-minium and its application [J]. Metallurgy Analysis, 1999, 19(1): 197-198.
Corresponding author: LIU Jian-hua; Tel/Fax: +86-10-82317103; E-mail: liujh@buaa.edu.cn


