
High temperature performance of
arc-sprayed aluminum bronze coatings for steel
ZHANG Zhong-li(张忠礼)1, LI De-yuan(李德元)1, WANG Shui-yong(王水勇)2
1. School of Materials Science and Engineering, Shenyang University of Technology, Shenyang 110023, China;
2. State Key Laboratory for Corrosion and Protection, Institute of Metal Reseach, Shenyang 110015, China
Received 22 February 2006; accepted 4 April 2006
Abstract:
The high-temperature oxidation behavior of arc-sprayed aluminum bronze coatings on steel substrate was studied during isothermal exposures in air at 900 ℃. The surface morphologies and interface of the coatings after isothermal oxidation at 900 ℃ for different times were observed. The experiments showed that the coatings on steel substrate were not deteriorated and the substrate was protected well, being exposed to high temperatures up to 900 ℃. The coatings withstood more than ten times thermal shock tests without any coating separation. The thermal expansion coefficient of the coatings was measured, revealing not much difference between it and that of steel substrate. After exposure at high temperature, the coatings were still adhered to steel substrate well. Isothermal mass gain of the coatings at elevated temperature in dry air was measured by means of a thermal balance and the oxidation behavior was evaluated by oxidation kinetic curves, exhibiting the oxidation kinetics curve accorded with a parabolic law. The parabolic rate constant of the oxidation kinetic curve is 1.02×10-9 g2?cm-4?s-1 for the first 60 min and from 150 min to 2 880 min the constant is 5.1×10-12 g2?cm-4?s-1.
Key words:
aluminum bronze; coatings; arc spray; high temperature oxidation;
1 IntroductionThe aluminum bronze coatings on steel substrate which are sprayed by electric arc process have been developed as possible new candidates for the use to high temperature applications, while their corrosion properties at high temperature in oxidizing atmospheres are not yet studied or are rarely investigated. This work is concerned with them.
Thermal sprayed coatings are widely used in corrosion and wear protection. However, the coating materials applied to high temperature are often ceramics or Ni-based alloys which are usually sprayed by a plasma process[1-4]. Though the plasma-sprayed coatings are competent for the applications, great operating costs and difficulty in working on site are two of the disadvantages. Compared with plasma process, arc spray has the advantages of low cost, high deposition rate and easy operating and there has been a growth of interest in the process. When the aluminum bronze is sprayed by the electric arc process, metallurgical interaction between sprayed particles and steel substrate could occur. This inherent feature of this process makes the arc sprayed aluminum bronze coatings have high bond strength. The coatings can strongly adhere to the smooth and clean surface of steel substrate by way of metallurgical interaction[5]. For this reason, aluminum bronze is widely used as one of the important bond coating materials when sprayed by the electric arc process[6, 7].
Aluminum bronzes are a family of copper-base alloys containing approximately 5% to 12% aluminum, some having additions of other elements. They have excellent corrosion resistance under a wide range of service conditions[8-10]. Moreover, it has been found in this study that the aluminum bronze coatings could prevent steel substrate from oxidation at elevated temperatures. The excellent bond ability combined with their good resistance to oxidation together with low cost and high deposition rate may make the aluminum bronze coatings promising in anti-oxidation applications. In order to make clear the behavior of the arc sprayed aluminum bronze coatings at elevated temperature, oxidation kinetics, thermal expansion coefficient and tensile adhesion strength of them were investigated. Also, the surface morphologies and interface of the coatings after isothermal oxidation at 900 ℃ for different time were observed.
2 Experimental
All the substrate of specimens was made of mild steel. The specimens were used for high temperature oxidation test and for thermal shock test. The dimension of thermal oxidation test specimens was 60 mm×40 mm×4 mm and the specimen preparation of tensile test was according to GB8642—88. The material used for arc spraying was a kind of aluminum bronze containing about 9.1%(mass fraction, the same below if not mentioned) Al and 2.1% Mn, which was used in the shape of d 3 mm wire. After being grit blasted, the specimens were sprayed with 0.6 mm aluminum bronze coatings in thickness. The parameters for arc spray process are listed in Table 1.
Table 1 Arc spraying parameters

The specimens for measuring thermal expansion coefficient of the coatings were tube-like. The specimen is illustrated in Fig.1. The steps are as follows: 1) making a brass mandrel of 2 mm diameter; 2) arc spraying the coatings on the mandrel to a diameter above 4.4 mm; 3) machining the sprayed mandrel to outer diameter of 4 mm; 4) drilling out the mandrel with great care and 5) cutting the tube-like specimen to length of 25 mm. The coefficients of linear expansion of the coatings were obtained by using a thermal dilatometer (produced by Rigaku Industrial Corporation).
![]()
Fig.1 Specimen for thermal expansion measurement
Thermal shock test was as following. Firstly, the specimens were heated to 900 ℃ and held at the temperature for 20 min, and then they were quenched into cool water. The thermal shock test was made for ten times to each specimen in the work. The isothermal oxidation tests were carried out at 900 ℃ in air at atmospheric pressure. The test temperature was held at 900 ℃, and the specimens were periodically removed from the furnace after 1, 5, 24 and 48 h. Then the surface and cross-section of the specimens were examined with scanning electron microscope(SEM) and electron probe micro analyzer(EPMA). For the tensile test, three groups of specimens were prepared for different test time and there were three specimens in a group. Group one was used for measuring the bond strength of the coatings as sprayed. Group two and group three were respectively for those after 1 h isothermal oxidation test and 24 h isothermal oxidation test. In addition, the oxidation kinetics of the coatings at 900 ℃ for 48 h was studied by using Cahn thermal balance.
In order to determine the oxide in the coatings, we dissolved two pieces of coating samples, one was as- sprayed and the other was oxidized under the test conditions for 48 h, in the solution of 5 mol H2NO3. The deposited materials were collected for X-ray diffraction analysis.
3 Results and discussion
The structure of aluminum bronze coatings sprayed by arc process is layer-like, which is composed of the alloy droplets, oxides and some pore spaces. During arc spraying process, alloy droplets are overheated and partly oxidized in air because temperature of arc is higher than 5 000 ℃[11]. There are a lot of oxides existing on the surface and inside the coatings and the coatings have a layer-like structure because the droplets strike against the substrate and become flat. The coatings are made of numerous particles covered with thin oxide film, which are mainly alumina. The microstructure of a cross section of the coatings and interface, along with their EMPA analyses, is shown in Fig.2. The oxide film on the particle surface may play a role equal to that of preoxidation. The oxide film acts as a barrier to the environment and the initial rate of metal degradation is low. When the coatings are heated, at first there are some other oxides formed such as copper oxide. As aluminum diffuses to the surface from inside the droplet, the oxides generally reduce to elements by aluminum because the formation of alumina will has lower Gibbs energy[12].
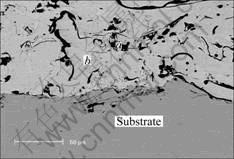
Fig.2 Microstructure of as-sprayed aluminum bronze coatings
Apart from oxides, there are also lots of pore spaces in arc-sprayed coatings. It is generally agreed that the presence of open pores within the coating structure is a main drawback of arc spray[13,14]. Pore spaces present in coatings allow the penetration of oxygen, which results in oxidation inside the coatings. It was found that the coatings contained more oxide after than before oxidation. According to Wagner oxidation theory, the oxidation is controlled by diffusion of elements in the oxide. When all the pore spaces closed, the oxides in the coatings would act as barriers to hinder oxygen or metal elements from diffusing to avoid inner alloy oxidizing. And diffusion of elements in the oxide became harder and harder, resulting in a reduced oxidation rate.
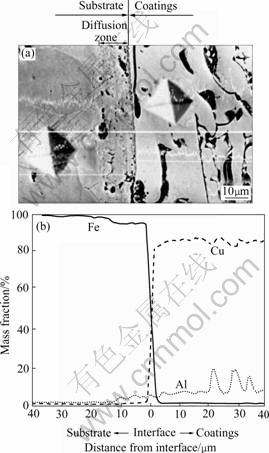
Fig.3 SEM micrograph and element distribution of coatings/ substrate interface heated at 900 ℃ for 1 h: (a) SEM micro- graph of interface; (b) Element distribution across interface
After the specimens were exposed in the furnace at 900 ℃ for 1 h, it was found that aluminum diffused from the coatings into the substrate (Fig.3), and that the bond strength values between aluminum bronze coatings and the steel substrate increase slightly (Table 2). However, with the heating time prolonged, the diffused zones were less and more oxides appeared at the coatings/substrate interface. Finally, a continuous oxide layer formed there after the test had lasted for more than 24 h. The results of adhesion test showed that the bond strength values between aluminum bronze coatings and the substrate did not decrease (Table 2). The usability to estimate a protective coating system includes that the coatings should adhere to the protected substrate reliably besides their excellent oxidation resistance. Though there was a continual oxide layer between the coatings and the steel substrate the test results revealed that the coatings were safely adhered to the substrate. This demonstrates that the oxide layer connected the coatings and the substrate with a chemical bond. When the test lasted to 48 h, no obvious coating spallation and failure was found, and the coatings protected the steel substrate against oxidation reliably. Fig.4 shows the SEM micrograph of interface after heated at 900 ℃ for 48 h.
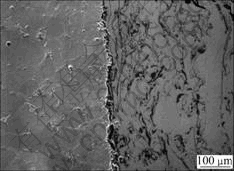
Fig.4 SEM micrograph of interface after heated at 900 ℃ for 48 h
Table 2 Bond strengths between aluminum bronze coatings and steel substrate(MPa)

The coatings also exhibited very good thermal shock resistance, which withstood ten cycles of 900 ℃ heating and water quenching without any coating separation. The test results also give evidence that there is a good adhesion between the coatings and the substrate. The thermal expansion coefficient of the aluminum bronze coatings in the temperature range from 100 ℃ up to 900 ℃ is measured and shown in Fig.5. The thermal expansion coefficient of mild steel is also given for comparison. From Fig.5 it can be seen that the thermal expansion coefficient of the coatings firstly increases with the temperature going up to 700 ℃ and then decreases, but the difference between the expansion coefficient of coatings and mild steel basically keep constant, and this prevents the separation between coatings and substrate. After the thermal shock test, some capillary cracks were found on the surface of the coatings, and the surface morphology and cross section microstructure observation revealed that most of the cracks had extended through the coatings and finally ended up at the substrate. The influence of the cracks on the oxidation resistance of the coatings will be investigated later.
Fig.6 shows the isothermal oxidation kinetics curve of aluminum bronze coatings heated at 900 ℃ for 48 h in air, which was obtained in the thermal oxidation test.
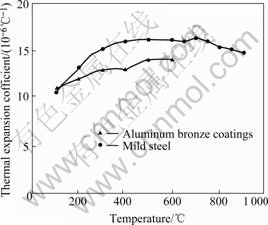
Fig.5 Thermal expansion coefficient of coatings
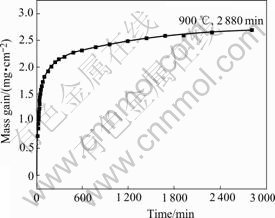
Fig.6 Oxidation kinetic of coatings oxidized in air at 900 ℃ for 48 h
It can be seen from Fig.6 that the oxidation kinetics curve accords with the parabolic law. The parabolic rate constant is 1.02×10-9 g2?cm-4?s-1 for the first 60 min and from 150 min to 2 880 min the constant is 5.1×10-12 g2?cm-4?s-1, which is much less than the former. At the early stage, because the pore spaces were easer paths for the entrances of oxygen, the oxidation took place more quickly. When most pore spaces were sealed by the oxides, diffusion of elements in the oxide became harder and harder, resulting in a reduced oxidation rate. The reason why the coatings show excellent oxidation- resistance is considered to be that an alumina-rich protective film exists. Fig.7 shows the surface morphology of the coatings after heated at 900 ℃ for 1 h. The color of oxide film on the coatings was yellow. The aluminum bronze coatings exhibited better oxidation resistance ability than the bulky aluminum bronze material at elevated temperatures. The reason is that the development of oxide spallation on the surface of the coatings is more difficult.
By means of chemical analysis, we knew that there was 7.21% Al left in the coatings as sprayed, which was enough to form a continual film rich in alumina on the surface[15]. The X-ray diffraction pattern of the oxide from the sprayed coatings is shown in Fig.8(a). Fig.8(b) shows the pattern of the oxide from the coatings specimen after oxidation test for 48 h. The analysis results indicated that the oxides in the as-sprayed coatings were mainly composed of γ-Al2O3 and the oxides in the heated sample were γ-Al2O3 and α-Al2O3. During spraying, the temperature of the particles decreased very rapidly and the rate of cooling is more than ten thousand degree centigrade per second[16]. Because of the high rate of transforming, a metastable phase, γ-Al2O3 was formed. And heating at 900 ℃ hardly made γ-Al2O3 transfer into α-Al2O3 so α-Al2O3 existing in the coatings could come from the process of post heating. Alumina film can provide an excellent protection on copper alloy base[17]. So, it is clear that alumina both on and inside the coatings plays the same role.
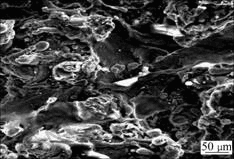
Fig.7 Surface morphology of coatings heated at 900 ℃ for 1 h
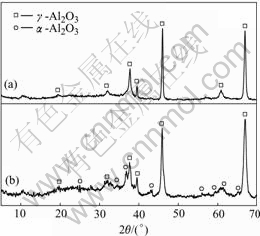
Fig.8 X-ray diffraction patterns of oxide: (a) As-sprayed; (b) After heating
4 Conclusions
Arc-sprayed aluminum bronze coatings can reliably bond to steel substrate and withstand 900 ℃ exposing without any obvious failure. The oxidation behavior of the coatings indicates that protective alumina-rich film, formed in arc spray process and in post-heating process, plays an important role in slowing down further oxidation rate. Alumina formed in the coatings seals the pore spaces of coatings during heating process. The special structure of the coatings makes them have better protectiveness. Oxidation kinetics of the coatings at 900 ℃ follows a parabolic law, indicating that the oxidation is governed by elementary diffusion. Together with the good bonding property, the good oxidation resistance will make aluminum bronze a promising coating material on steel for high temperature service.
References[1] LIU Pei-sheng, LIANG Kai-ming, ZHOU Hong-yu. High temperature protective coatings on superalloys [J]. Trans Nonferrours Met Soc China, 2002, 12(4): 798-803.
[2] SIDHU BS, PRAKASH S. High-temperature oxidation behavior of NiCrA1Y bond coats and Stellite-6 plasma-sprayed coatings [J]. Oxidation of Metals, 2005, 63(3/4): 241-259.
[3] NIRANATLUMPONG P, PONTON CB, EVANS HE.The failure of protective oxides on plasma-sprayed NiCrAlY overlay coatings [J]. Oxidation of Metals, 2000, 53(3/4): 241-258.
[4] ITOH Y, SAIOH M, ISHIWATA Y. Influence of high-temperature protective coatings on the mechanical properties of nickel-based superalloys [J]. Journal of Materials Science, 1999, 34(16): 3957-3966.
[5] WEN Jin-lin, ZHANG Zhong-li. An investigation of the self-bonding mechanism of aluminum bronze coatings sprayed by the electric arc process [A]. Proceedings of 11th International Thermal Spraying Conference [C]. Montreal, 1986. 169-175.
[6] ZHANG Zhong-li, HAO Yan-ping. Comparison of arc sprayed bond coatings [A]. WEI Zheng. Proceedings of National Thermal Spraying Conference [C]. Shenyang, 1999. 57-63. (in Chinese)
[7] LIU Ai-hua, ZHANG Zhong-li. A new kind of self-bonding coatings [J]. Journal of Senyang University of Technology, 1998, 20(4): 61-65. (in Chinese)
[8] CAMPBELL H S. Aluminum bronze alloys corrosion resistance guide [M]. London: CDA (UK) Publication, 1981.
[9] CANEY R J T. Corrosion resistant aluminium bronze [J]. Australasian Engineer, 1954, 46: 54-69.
[10] LU Yang, YUAN Li-hua, LI Wen-sheng. Study of corrosion behavior high strength and wear-resistant aluminum bronze in 10% H2SO4 solution [J]. Journal of Lanzhou University of Technology, 2005, 31(4): 31-34. (in Chinese)
[11] STEEPER T J, IRONS G, KRATOCHVIL W R. A Taguchi experimental design study of twin-wire electric arc sprayed aluminum coatings [A]. BERNDT C C. Proceedings of 13th International Thermal Spraying Conference [C]. USA: Orlando, 1992. 427-432.
[12] ZHOU Chun-gen, XU Hui-bin, GONG Sheng-kai. Investigation on oxidation behavior of Al-Cu-Fe quasi-crystal [J]. Chinese Journal of Aeronautics, 2001, 14 (3): 178-182.(in Chinese)
[13] ROCHA A C, RIZZO F. Duplex Al-based thermal spray coatings for corrosion protection in high temperature refinery applications [J]. Materials Research, 2004, 7(1): 1-6.
[14] KULKARNI AA, SAMPATH S, GOLAND A. Plasma spray coatings for producing next-generation supported membranes [J]. Topics in Catalysis, 2005, 32(3/4): 241-249.
[15] KAI W, FAN G W, CHEN P C. The corrosion of Cu-Al binary alloys in H2/H2S/H2O atmospheres at 400-900℃ [J]. Oxidation of Metals, 2004, 61(5/6): 439-461.
[16] MOREAU C, CIELO P, LAMONTAGNE M. Flattening and Solidification of Thermal Sprayed Particles [A]. BERNDT C C. Proceedings of 13th International Thermal Spraying Conference [C]. Orlando, 1992. 761-766.
[17] DUO Shu-wang, LI Mei-shuan, QIAN Yu-hai. Protection of copper from atomic oxygen by copper based Al2O3 coatings [J]. The Chinese Journal of Nonferrous Metals, 2003, 13(1): 172-176. (in Chinese)
(Edited by LONG Huai-zhong)
Corresponding author: ZHANG Zhong-li; Tel: +86-24-25691331; E-mail: zhonglil@yahoo.com


