文章编号:1004-0609(2011)06-1308-06
AZ31与NZ30K合金作为镁电池负极材料的电化学性能
周丽萍1,曾小勤1, 2,常建卫1,刘 志1,朱亚琪1,姚 嵩1,丁文江1, 2
(1. 上海交通大学 材料科学与工程学院 上海市镁材料及应用工程技术研究中心,上海 200240;
2. 上海交通大学 材料科学与工程学院 金属基复合材料国家重点实验室,上海 200240)
摘 要:
采用恒温浸泡、交流阻抗和极化曲线法分别研究铸态(F)和固溶态(T4)的NZ30K以及挤压态AZ31镁合金在不同浓度MgCl2、MgSO4、Mg(COOCH3)2、MgBr2溶液中的腐蚀行为和电化学性能。结果表明:随着电解液中电解质浓度的增加,3种镁合金的自腐蚀速率均增大。F态和T4态的NZ30K合金在MgSO4溶液中腐蚀速率最快,在MgBr2溶液中耐蚀性能最好,而AZ31合金在MgCl2溶液中耐蚀性能最差,在MgSO4和Mg(COOCH3)2中具有较好的耐蚀性能。电化学阻抗谱(EIS)结果表明:在4种电解液中,镁合金的高频端容抗环半径均随着电解质浓度的增加而减小,这与恒温浸泡的实验结果相吻合。
关键词:
中图分类号:O646.6 文献标志码:A
Electrochemical properties of AZ31 and NZ30K alloys as anode materials for magnesium battery
ZHOU Li-ping1, ZENG Xiao-qin1, 2, CHANG Jian-wei1, LIU Zhi1, ZHU Ya-qi1, YAO Song1, DING Wen-jiang1, 2
(1. Shanghai Engineering Research Centre of Magnesium Materials and Application,
School of Materials Science and Engineering, Shanghai Jiao Tong University, Shanghai 200240, China;
2. Key State Laboratory of Metal Matrix Composite, School of Materials Science and Engineering,
Shanghai Jiao Tong University, Shanghai 200240, China)
Abstract: The corrosion behavior and the electrochemical performance of NZ30K alloy of as-cast(F) and solution-treatment conditions(T4), and AZ31 alloy of as-extruded condition were investigated in MgCl2, MgSO4, Mg(COOCH3)2 and MgBr2 solution with different molar concentrations by means of immersion test at constant temperature, alternating current impedance and polarization curve. The results show that the corrosion rates of three kinds of Mg alloys increase with the increase of the electrolyte concentration. The corrosion rate of NZ30K alloy in F and T4 conditions in MgSO4 solution is the highest, and that in MgBr2 solution among the researched electrolytes is the lowest. Whereas, AZ31 alloy shows the worst corrosion resistance in MgCl2 solution and the best corrosion resistance in MgSO4 and Mg(COOCH3)2 solution. The results of electrochemical impedance spectroscopy(EIS) show that radius of high frequency capacitive loop of Mg alloys decreases with the increase of the electrolyte concentration, which is consistent with the results of immersion test at constant temperature.
Key words: AZ31 alloy; NZ30K alloy; corrosion behavior; electrochemical performance
镁是活泼金属,电负性为1.31,标准电极电位为-2.36 V(vs SHE),理论比容量高达2.20 A×h/g,在常见的金属中仅比锂(3.86 A×h/g)、铝(2.98 A×h/g)小,远大于锌(0.82 A×h/g)。因此,镁是一种很有前途的电池负极材料[1-3]。镁合金作为电池负极材料的主要障碍之一是镁合金与电解液的匹配问题。在中性和酸性条件下,镁与水剧烈反应放出氢气,使电极利用率降低[4];碱性条件下,镁合金表面会形成致密的保护膜而使电极钝化,阻止放电的持续进行。另外,镁在放电时存在比较严重的负差效应,降低了电流效率[5-7]。为解决这些问题,途径之一是开发合适的镁合金种类,使其具有较高的放电电流效率。如SIVASHANMUGAM等[8]合成的Mg-Li合金,Li含量占13%(质量分数),由该合金组装的Mg-Li/MgCl2/CuO电池具有开路电位高的优点,在8.6 mA/cm2电流密度下,阳极电流效率能够达到81%[9]。另外,寻找合适的电解液体系并辅以一定的缓蚀剂也是改善镁电池性能的一个途径,但目前仍未有关于此方面系统性研究的报道。
本文作者对比研究铸态(F)、固溶态(T4)的NZ30K以及挤压态AZ31镁合金在不同浓度MgCl2、MgSO4、Mg(COOCH3)2、MgBr2溶液中的腐蚀行为和电化学 性能。
1 实验
NZ30K和AZ31镁合金的组成分别为(质量分数):3% Nd、0.2% Zn、0.4% Zr、余量为Mg;3% Al、1% Zn、0.2% Mn、余量为Mg。4种电解液分别由分析纯MgCl2、MgSO4、Mg(COOCH3)2、MgBr2溶于蒸馏水而得。
自腐蚀速率测试采用水浴恒温浸泡试验,试样尺寸为20 mm×20 mm×5mm,电解液体积为200 mL,电解质测试温度为30 ℃,浸泡时间为18 h。电化学测试仪器为PARSTAT 2273电化学工作站。分别以石墨作辅助电极,饱和KCl甘汞电极作参比电极,组成三电极体系,测定电极材料的电化学性能[10]。工作电极的制备方法:将镁合金线切割加工成d 12 mm、厚度为5 mm的圆片,将测试面经砂纸细磨后,用金刚石研磨膏抛光,经蒸馏水和酒精清洗后吹干待用[11]。
准确测出镁合金试样的初始质量m0(mg)和面积S(cm2),将其悬挂于200 mL电解液中,使其不与烧杯底部接触。18 h后取出先用自来水冲洗,再用蒸馏水清洗并热风吹干,随后放入65 ℃的200 g/L铬酸溶液中浸泡5 min,取出后用蒸馏水清洗,热风吹干,称取质量m1(mg)[12],自腐蚀速率v按式(1)计算[13-14]:
![]() (1)
(1)
式中:v为自腐蚀速率,mg?cm-2?h-1;t为浸泡时间,h;S为试样表面积,cm2。
电化学测试前将工作电极经砂纸打磨后,用金刚石研磨膏抛光,依次用蒸馏水和酒精冲洗干净后用热风吹干。工作电极浸入电解液后立即进行开路电位测试,测试时间为3600 s。开路电位测试结束后再进行电化学阻抗谱测试,频率范围为0.1 Hz~0.1 MHz,交流幅值为10 mV。动电位极化曲线测试在电化学阻抗谱测试结束后进行,扫描速率为1 mV/s,起始电位(φ0)为相对于开路电位负250 mV,腐蚀电流迅速增大时的腐蚀电位(φ1)为终止电位[15]。
2 结果与分析
2.1.1 腐蚀表面形貌
图1所示为铸态(F)和固溶态(T4)NZ30K合金以及挤压态的AZ31合金在浓度为1.5 mol/L的MgCl2、MgSO4、Mg(COOCH3)2、MgBr2溶液中浸泡18 h并洗去腐蚀产物后试样的表面形貌。由图1可以看出,F态和T4态NZ30K合金在MgSO4溶液中腐蚀最严重,在MgBr2溶液中耐蚀性最好;而AZ31合金在MgCl2溶液中耐蚀性能最差,在MgSO4和Mg(COOCH3)2溶液中具有较好的耐蚀性能。
2.1.2 自腐蚀速率
图2所示是恒温浸泡后F态、T4态NZ30K和AZ31合金的自腐蚀速率。由图2可以看出,对于3种状态的镁合金,自腐蚀速率均随着电解液中电解质浓度的增加而增大,而T4态NZ30K合金在电解质浓度为2 mol/L的Mg(COOCH3)2溶液中自腐蚀速率相对于1.5 mol/L的电解液中的略有降低。从图2(a)和(b)可看出,对于同一浓度的电解液,F态和T4态的NZ30K合金在MgSO4溶液中的自腐蚀速率最大,在MgBr2溶液中的自腐蚀速度最小。从图2(c)可看出,AZ31镁合金在MgCl2溶液中耐蚀性能最差,在MgSO4和Mg(COOCH3)2中具有较好的耐蚀性能[16]。
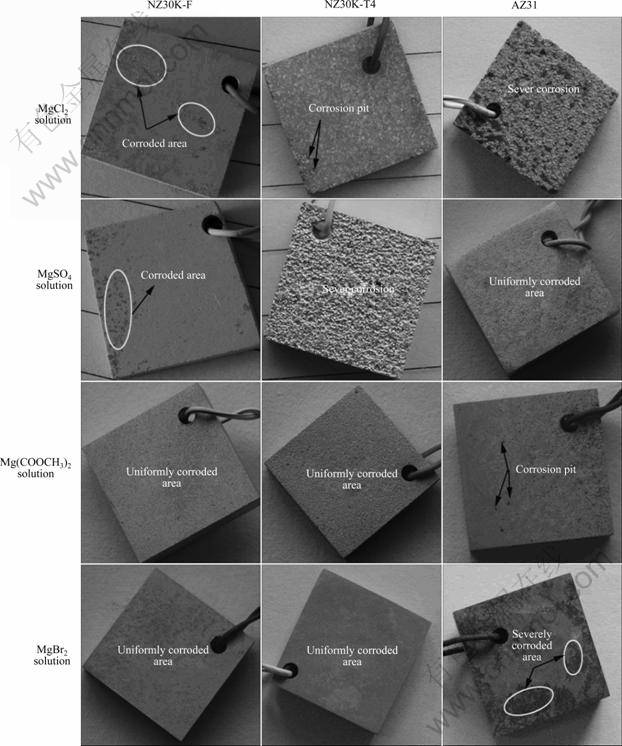
图1 F态和T4态NZ30K以及AZ31合金在电解液中浸泡18 h后的腐蚀表面照片
Fig.1 Corroded surface photographs of F and T4 states NZ30K and AZ31 alloys after immersion in electrolytes for 18 h and removal of corrosion products
2.2.1 开路电位
图3所示为F态、T4态NZ30K和AZ31合金在1.5 mol/L的MgCl2溶液中电位随时间的变化曲线。从图3可以看出,AZ31合金的腐蚀电位最高。对于干净的试样表面,在开始阶段500 s内,3种合金的腐蚀电位均随时间增加而快速升高,然后随着时间的增加而缓慢升高。这是由于镁合金试样在浸入电解质溶液的初期,发生析氢反应,腐蚀产物膜形成速度较快,金属表面被膜层覆盖,开路电位逐渐上升,当镁合金表面被膜层完全覆盖后,镁合金表面成膜过程与膜溶解过程达到动态平衡,开路电位几乎不发生变化,达到稳定。浸泡1 h后,AZ31合金的腐蚀电位为-1.61 V (vs SCE),而NZ30K-F和NZ30K-T4合金的腐蚀电位分别为-1.75和-1.78 V (vs SCE)。
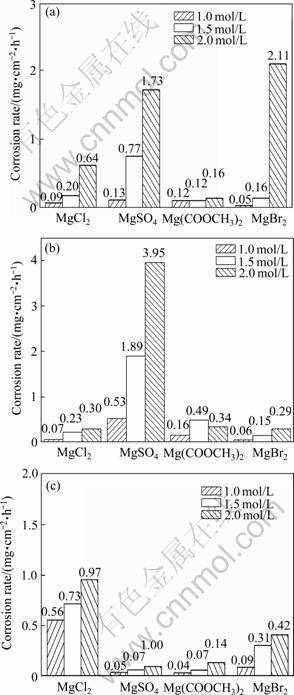
图2 NZ30K-F、NZ30K-T4和AZ31合金在电解液中的腐蚀速率
Fig.2 Corrosion rates of NZ30K-F(a) and NZ30K-T4(b) and AZ31 (c) after immersion in electrolytes at constant temperature
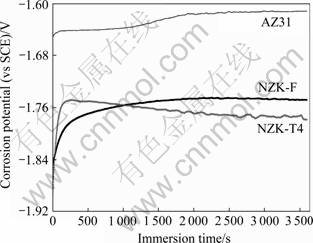
图3 NZ30K-F、NZ30K-T4和AZ31合金在1.5 mol/L MgCl2电解液中的腐蚀电位
Fig.3 Corrosion potentials of NZ30K-F, NZ30K-T4 and AZ31 alloys after immersion in 1.5 mol/L MgCl2 solution
2.2.2 动电位极化曲线
图4所示为F态NZ30K合金在不同浓度MgBr2溶液中浸泡1 h后的动电位极化曲线。表1所列为从图4得到的NZ30K-F合金的腐蚀电位(φcorr)和腐蚀电流密度(Jcorr)值。由图4可看出,随着电解液中电解质浓度的增加点蚀电位(φpt)几乎不变,为-1.61 V。腐蚀电流密度(Jcorr)随着电解液中电解质浓度增加而增大,当电解液浓度增加到2.0 mol/L时,腐蚀电流密度增大幅度很大,达到682.24 μA/cm2,比浓度为1.0和1.5 mol/L时高一个数量级。这说明合金在浓度为2.0 mol/L的MgBr2溶液中腐蚀现象严重,这与图2(a)自腐蚀速率结果一致。从表1可以看出,合金腐蚀电位(φcorr)随电解液浓度增加而正移。
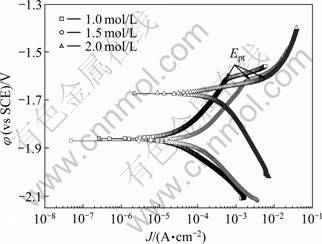
图4 NZ30K-F合金在不同浓度MgBr2溶液中的极化曲线
Fig.4 Polarization curves of NZ30K-F alloy in MgBr2 solution with different concentrations
表1 NZ30K-F合金在不同浓度MgBr2溶液中的Jcorr和φcorr
Table 1 Jcorr and φcorr values of NZ30K-F alloy in MgBr2 solution with different concentrations

F态、T4态NZ30K和AZ31合金在1.5 mol/L的MgSO4溶液中的极化曲线如图5所示。表2所列为由图5动电位极化曲线得到的腐蚀电流密度和腐蚀电位。由图5和表2可以看出,AZ31合金Jcorr值最小,比F态NZ30K合金小一个数量级,比T4态NZ30K小两个数量级,这与图2的自腐蚀速率结果相吻合。
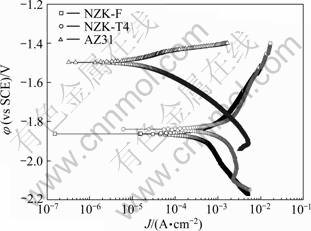
图5 NZ30K-F、NZ30K-T4和AZ31合金在1.5 mol/L MgSO4溶液中的极化曲线
Fig.5 Polarization curves of NZ30K-F, NZ30K-T4 and AZ31 alloys in 1.5 mol/L MgSO4 solution
表2 NZ30K-F、NZ30K-T4和AZ31合金在1.5 mol/L MgSO4溶液中的Jcorr和φcorr
Table 2 Jcorr and φcorr values of NZ30K-F, NZ30K-T4 and AZ31 alloys in 1.5 mol/L MgSO4 solution

2.2.3 电化学阻抗谱
图6所示为F态和T4态NZ30K合金在不同浓度MgBr2溶液中的电化学阻抗谱。由图6可看出,两种状态的NZ30K合金的EIS谱形状相似,表明两种状态合金的腐蚀机理相同。两种状态合金的阻抗谱均由两个容抗环组成,高频端和中频端的是由电荷传递引起,其直径可以近似看作电极反应电荷传递电阻;低频端的容抗环由吸附在镁合金表面的物质驰豫过程引起,可看成膜电阻。随着电解液中电解质浓度增加,高频端和中频端容抗环半径减小,合金耐蚀性变差。
AZ31镁合金在不同浓度Mg(COOCH3)2溶液中的交流阻抗谱如图7所示。由图7可知,镁合金的高频容抗环半径随着电解液中电解质浓度的增加而减小,且当浓度超过1.0 mol/L时,高频端的阻抗急剧减小,这主要是因为浓度增加时,电解液与合金的电化学反应阻力降低。这与恒温浸泡的实验结果相吻合。

图6 NZ30K-F和NZ30K-T4合金在不同浓度MgBr2溶液中的阻抗谱
Fig.6 Nyquist curves of NZ30K-F (a) and NZ30K-T4 (b) alloys in MgBr2 solution with different concentrations
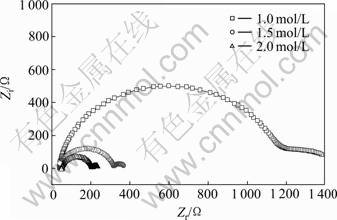
图7 AZ31合金在不同浓度Mg(COOCH3)2溶液中的阻抗谱
Fig.7 Nyquist curves at free immersion potential of AZ31 alloy in Mg(COOCH3)2 solution with different concentrations
3 结论
1) F态和T4态的NZ30K合金在MgSO4溶液中腐蚀速率最快,在MgBr2溶液中耐蚀性能最好,而AZ31合金在MgCl2溶液中耐蚀性能最差,在MgSO4和Mg(COOCH3)2中具有较好的耐蚀性能。
2) 3种合金的腐蚀电位和腐蚀电流密度均随着电解液浓度的增加而增大,合金的腐蚀电流密度变化与恒温浸泡实验结果相一致。
3) 在所研究的4种电解液中,镁合金的高频端容抗环半径均随着电解质浓度的增加而减小,且当溶度超过1.0 mol/L时,高频端的容抗环半径急剧减小。
REFERENCES
[1] UDHAYAN R, BHATT P D. On the corrosion behaviour of magnesium and its alloys using electrochemical techniques[J]. Journal of Power Sources, 1996, 63(1): 103-107.
[2] 唐有根, 黄伯云, 卢凌彬, 刘东任. 金属燃料电池[J]. 物理, 2004, 33(2): 85-89.
TANG You-gen, HUANG Bai-yun, LU Ling-bin, LIU Dong-ren. Metal fuel cells[J]. Physics, 2004, 33(2): 85-89.
[3] BALASUBRAMANIAN R, VELUCHAMY A, VENKATAKRISHNAN N, GANGADHARAN R. Electrochemical characterization of magnesium/silver chloride battery[J]. Journal of Power Sources, 1995, 56(2): 197-199.
[4] 程英亮, 陈振华, 王慧敏, 吴有伍. 镁合金的腐蚀与防护研究进展[J]. 机械工程材料, 2005, 29(5): 1-4.
CHENG Ying-liang, CHEN Zhen-hua, WANG Hui-min, WU You-wu. Progress in the research of corrosion and protection of magnesium alloys[J]. Materials for Mechanical Engineering, 2005, 29(5): 1-4.
[5] SONG Guang-ling, ATRENS A, WU Xian-liang, ZHANG Bo. Corrosion behavior of AZ21, AZ501 and AZ91 in sodium chloride[J]. Corrosion Science, 1998, 40(10): 1769-1791.
[6] 李 瑛, 宋光铃, 林海潮, 曹楚南. 金属镁在腐蚀介质中界面结构特征与负差数效应关系研究[J]. 腐蚀科学与防护技术, 1999, 11(4): 202-208.
LI Ying, SONG Guang-ling, LIN Hai-chao, CAO Chu-nan. Study on the relationship between the corrosion interface structure and negative difference effect for pure magnesium[J]. Corrosion Science and Protection Technology, 1999, 11(4): 202-208.
[7] 徐卫军. 镁合金腐蚀的负差数效应[J]. 甘肃联合大学学报, 2007, 21(3): 44-46.
XU Wei-jun. The negative difference effect in corrosion of magnesium alloys[J]. Journal of Gansu Lianhe University, 2007, 21(3): 44-46.
[8] SIVASHANMUGAM A, KUMAR T P, RENGANAHAN N G. Performance of a magnesium-lithium alloy as an anode for magnesium batteries[J]. Journal of Applied Electrochemistry, 2004, 34(11): 1135-1139.
[9] 王宇轩, 李 林, 黄锐妮, 韩雪荣, 于 磊, 马正青. 合金元素Ga、X对镁基负极材料电性能的影响[J]. 电源技术, 2006, 30(12): 1003-1005.
WANG Yu-xuan, LI Lin, HUANG Rui-ni, HAN Xue-rong, YU Lei, MA Zheng-qing. Effect of alloying elements Ga and X on the electric properties of magnesium alloy anode materials[J]. Power Technology, 2006, 30(12): 1003-1005.
[10] CHANG Jian-wei, GUO Xing-wu, HE Shang-ming, FU Peng-huai, PENG Li-ming, DING Wen-jiang. Investigation of the corrosion for Mg-xGd-3Y-0.4Zr(x=6, 8, 10, 12 wt%) alloys in a peak-aged condition[J]. Corrosion Science, 2008, 50(1): 166-177.
[11] GUO Xing-wu, CHANG Jian-wei, HE Shang-ming, DING Wen-jiang, WANG Xi-shu. Investigation of corrosion behaviors of Mg-6Gd-3Y-0.4Zr alloy in NaCl aqueous solutions[J]. Electrochimica Acta, 2007, 52(7): 2570-2579.
[12] CHANG Jian-wei, FU Peng-huai, GUO Xing-wu, PENG Li-ming, DING Wen-jiang. The effects of heat treatment and zirconium on the corrosion behaviour of Mg-3Nd-0.2Zn-0.4Zr [wt.%] alloy[J]. Corrosion Science, 2007, 49(6): 2612-2627.
[13] 陈昌国, 司玉军, 余丹梅, 刘渝萍, 杨祖洪, 王 琪, 李 兰. AZ31 镁合金在MgSO4溶液中的电化学行为[J]. 中国有色金属学报, 2006, 16(5): 781-785.
CHEN Chang-guo, SI Yu-jun, YU Dan-mei, LIU Yu-ping, YANG Zu-hong, WANG Qi, LI Lan. Electrochemical behavior of AZ31 magnesium alloy in MgSO4 solution[J]. The Chinese Journal of Nonferrous Metals, 2006, 16(5): 781-785.
[14] 封雪松, 熊中平, 司玉军, 李敏娇. AZ31和AZ61镁合金在MgSO4溶液中的电化学行为对比[J]. 腐蚀与防护, 2007, 28(11): 553-555.
FENG Xue-song, XIONG Zhong-ping, SI Yu-jun, LI Min-jiao. Comparison of electrochemical behaviors of AZ31 and AZ61 magnesium alloys in MgSO4 solution[J]. Corrosion and Protection, 2007, 28(11): 553-555.
[15] BELDJOUDI T, ROBBIOLA L, FIAUD C. Influence of homogenization and artificial aging heat treatments on corrosion behavior of Mg-Al alloys[J]. Corrosion, 1993, 49(9): 738-745.
[16] 尧玉芬, 陈昌国, 刘渝萍, 王 荣. 镁电池的研究进展[J]. 材料导报, 2009, 23(10): 119-121.
YAO Yu-fen, CHEN Chang-guo, LIU Yu-ping, WANG Rong. Research progress of magnesium battery[J]. Materials Review, 2009, 23(10): 119-121.
(编辑 李艳红)
基金项目: 上海市科委重点专项资助项目(10dz2211000)
收稿日期:2010-06-28;修订日期:2010-09-20
通信作者:曾小勤,教授,博士;电话:021-54742301;E-mail: xqzeng@sjtu.edu.cn


