
Influence of high intensity ultrasonic vibration on microstructure of
in-situ synthesized Mg2Si/Mg composites
CHEN Ke(陈可), LI Zi-quan(李子全), ZHOU Heng-zhi(周衡志), WANG Wei(王伟)
College of Materials Science and Technology, Nanjing University of Aeronautics and Astronautics,
Nanjing 210016, China
Received 15 July 2007; accepted 10 September 2007
Abstract:
Coarse and agglomerated primary Mg2Si phase in in-situ synthesized Mg2Si/Mg composite with 4%Si was treated in remelting process by means of high intensity ultrasonic vibration. The effects of ultrasonic vibration duration and temperature on size, morphology and distribution of the primary Mg2Si were studied. The evolution mechanism was discussed. The microstructures of the composites were investigated by means of optical microscopy (OM) and scanning electronic microscopy (SEM). The components were inspected with energy dispersion spectrum (EDS) and X-ray diffraction (XRD). The results indicate that ultrasonic vibration does not alter two constituents of the composites, but changes the size and distribution of aggregated primary Mg2Si particles. The size of primary Mg2Si particles decreases with the increase of vibration duration and vibrating temperature. High intensity ultrasonic has little effects on the primary Mg2Si morphology. The high intensity ultrasonic vibration is an effective means to prepare well-proportioned in-situ synthesized magnesium matrix composites.
Key words:
Mg2Si/Mg composite; high intensity ultrasonic vibration; in-situ synthesis; microstructure; primary Mg2Si;
1 Introduction
Mg-Al-Si alloy, one of low-cost and heat-resistant Mg alloys, is widely applied in aeronautics, automobile and electronic industry, etc[1]. More attentions are paid for in-situ synthesis of Mg matrix composites based on the higher Si alloy system[2-3]. One of main reasons is that the in-situ synthesized Mg2Si phase, which is characterized by high melting point(1 080 ℃), high rigidity (HV460), low density(1.9 g/cm3) and low heat dilatability, can effectively improve the mechanical properties, wear resistibility and heat resistibility of the alloy[4]. However, coarse, inhomogenously distributed and partly agglomerated Mg2Si particles are easily present in the alloy with the increase of Si content[5-6], which results in a decrease of the mechanical properties (especially elongation), castabilities and processabilities of alloy. Recent research showed that modified elements, such as Y and B, can refine the primary Mg2Si in Mg-Si alloy[7]. But the distribution of primary Mg2Si in larger area wasn’t improved, so entirely modification routes are still in exploring.
High intensity ultrasonic processing is an effective way to prepare exogenous metallic matrix composites (MMC)[8-9]. The spread of ultrasound in melt will introduce the reinforcement particles into matrix melt. Moreover, it accelerates wetting and dispersed process, and facilitates degassing and deslaging from the melts. This directly results in the improvement of MMC performances and lower fabrication cost. Ultrasonic vibration is widely applied to Al alloy and particles reinforced MMC, while its application on Mg matrix composites is rare and attractive.
The main aims of the present work are to introduce high intensity ultrasonic into in-situ synthesized Mg2Si/Mg composites with 4%Si in remelting process, to explore effects of ultrasonic vibration duration and temperature on size, morphology and distribution of primary Mg2Si, and to reveal the evolution mechanism.
2 Experimental
Mg-4%Si alloy were firstly in-situ synthesized. Commerce Mg ingots were melted to above 650 ℃ and silicon was added into Mg melts. Then the melts were heated to above 800 ℃ and kept about 30 min to ensure Mg and Si fully reacted. When temperature dropped to under 750 ℃, melts were stirred for several minutes, and then cast into a stainless steel mold at 680 ℃. The whole smelting was protected with SF6+CO2 gas mixture.
Mg2Si/Mg composites were remelted to destination temperatures and kept for 30 min, then stirred by high energy ultrasonic for certain time. Ultrasonic frequency was 20-22 kHz and ultrasonic power was 1 kW. After ultrasonic vibration, sample 1-7 were got by sucking the melt into a steel tube and rapidly oil-chilled. Different ultrasonic vibration parameters of samples are listed in Table 1.
Table 1 Alloy number and corresponding ultrasonic vibration parameters
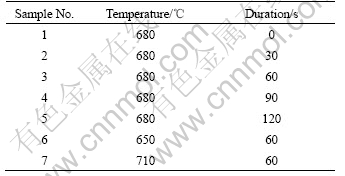
All the specimens were kibbled, fine ground and polished, then etched by 4% nitric acid ethanol solution. Bruker D8 advanced X-ray diffractometer was employed to analyze the phase structure. The microstructures of the alloys were analyzed with Leitz WETZLAR MM6 Optical Microscope (OM). DT2000 image analysis software is used to measure the average area of primary Mg2Si in every image. Energy dispersion spectrum (EDS) affiliated to the FEI QUANTA200 Scanning Electron Microscope (SEM) was employed to analyze the chemical compositions of phase in alloys.
3 Results and discussion
3.1 Influence of vibrating duration on Mg2Si/Mg composite
Fig.1 shows the microstructure of sample 1 (Mg2Si/Mg composite without ultrasonic vibration). The microstructure of alloy consists of two constituents: polygonal primary Mg2Si and Mg-Mg2Si eutectoid. Primary Mg2Si polygons are bad-proportioned (in Fig.1), and partial Mg2Si is congregated (as pointed by arrow). Eutectic Mg2Si presents bulky Chinese-character-like dendrites. EDS and XRD tests were carried out to ensure phase composition and structure (in Fig.2(b) and 3).
After ultrasonic vibrating, the constituents of alloys were unchanged, but the microstructure changed. With the vibrating durations increase from 30 to 120 s, primary Mg2Si polygons were apart from each other gradually (shown in Fig.4). Primary Mg2Si particles were well dispersed after vibration time reached 60 s. Average area of 50 Mg2Si particles were measured and its variation with vibrating time is shown in Fig.5. This shows that average size of Mg2Si particles decreases with the ultrasonic vibration. After vibrating for 60 s, prolonging the vibrating duration has neither obvious effects on the size nor on distribution of primary Mg2Si particles, but would increase risk of oxidizing. The Mg2Si morphology is only slightly changed.
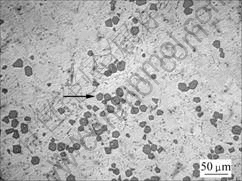
Fig.1 Microstructure of sample 1
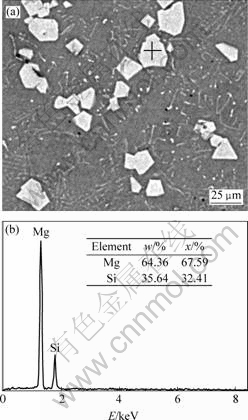
Fig.2 EDS pattern of point indicated by “+” in sample 2
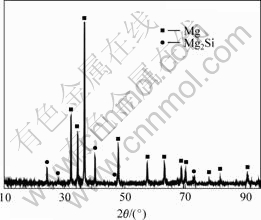
Fig.3 XRD pattern of sample 2
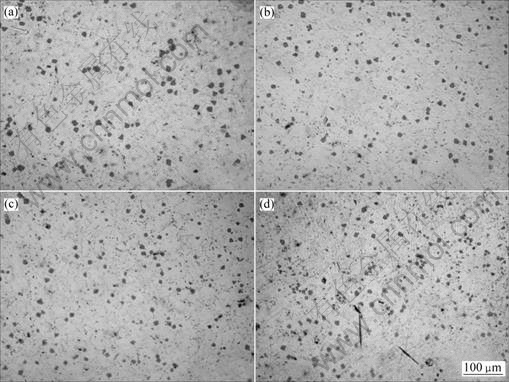
Fig.4 Microstructures of samples vibrated for different times: (a) 30 s (sample 2); (b) 60 s (sample 3); (c) 90 s (sample 4); (d) 120 s (sample 5)
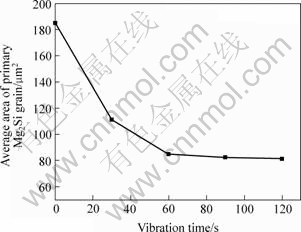
Fig.5 Relationship between vibration time and average area of primary Mg2Si grain
3.2 Influence of vibrating temperature to Mg2Si/Mg composite
Fig.4(b), Figs.6(a) and (b) show the microstructures of Mg2Si/Mg composites vibrated for 60 s at 680, 650 and 710 ℃, respectively. The test temperatures are below the liquidus temperature but above eutectic temperature. Fig.7 shows the average area of primary Mg2Si particles. This indicates that primary Mg2Si particles become smaller as the vibrating temperature increases from 650 to 710 ℃. Primary Mg2Si particles were kept in a well dispersed state and after vibration time reached 60 s. The Mg2Si morphology tends to sphere.
3.3 Discussion
It’s known that two basic acoustic effects, cavitation and acoustic streaming, will occur when high intensity ultrasound propagates in a liquid medium. These two effects greatly influence the evolution of Mg2Si.
The burst of cavitation bubbles in metal melt will produce shock wave, of which pressure could reach 105 MPa[10]. The congregated primary Mg2Si particles will be driven to be scattered at such a high pressure.
When ultrasound spread in liquid, capillarity in melt will be greatly enhanced. It’s conduced to infiltration of liquid to granule agglomeration. Critical osmotic pressure (ps) formula of pressed global granula is as follows[11]:
![]()
where dp is the average diameter of granula, σLG is the surface tension of liquid, φ is the volume fraction of interspace between granula, λ is geometry factor (generally take it as 1.4) and θ is the contact angle.
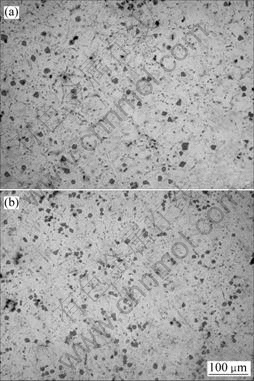
Fig.6 Microstructures of samples vibrated at different temperatures: (a) 650 ℃ (sample 6); (b) 710 ℃ (sample 7)
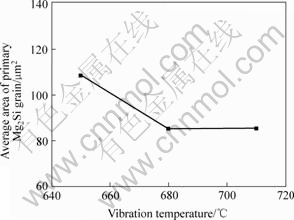
Fig.7 Relationship between vibration temperature and average area of primary Mg2Si grain
If the primary Mg2Si particle is regarded as global one (dp≈10 mm), and σLG=0.559 N/m[12], φ≈0.2, θ=180?, ps≈1.878×106 Pa. It’s much less than the pressure of shock wave that produced by burst of cavitation bubbles. So it’s easy for Mg melt to infiltrate primary Mg2Si particle agglomerate and separate particles from each other.
High pressure shock wave, melt flow and instantaneous local temperature of melts, resulted from the cavitation and acoustic streaming, enhance the dissolution of the primary Mg2Si. At a constant temperature, primary Mg2Si size is gradually refined. Moreover, dissolution and growth of Mg2Si can reach a dynamic balance after vibration for over 60 s. This results in primary Mg2Si size hardly changes and morphology is only slightly changed.
When vibration temperature is low, high volume fraction of solid in melt leads to the high melt viscidity which depresses the effect of ultrasonic vibration. A lower dissolution rate of Mg2Si results in a larger Mg2Si. At a higher vibrating temperature, intensive cavitation and acoustic streaming accelerate the dissolution of sharp-angle Mg2Si. The Mg2Si morphology tends to sphere.
4 Conclusions
1) Inhomogenously distributed primary Mg2Si particles in in-situ synthesized Mg2Si/Mg composite are refined and well-dispersed by introduction of high intensity ultrasonic vibration. The refined effect enhances with the increase of vibration time and vibrating temperature. High intensity ultrasonic has little effects on the primary Mg2Si morphology.
2) High pressure of shock wave caused by burst of cavitation bubbles and enhanced capillarity effect are main reasons for dispersion of primary Mg2Si granula. Melt flow and instantaneous local temperature of melts enhance the dissolution of the primary Mg2Si, but dissolution and growth of Mg2Si can reach a dynamic balance after a certain time vibration.
References
[1] HOLLRIGL-ROSTA F, JUST E, KOHLER J. Magnesium in the Volkswagen[J]. Light Metal Age, 1980(4): 22.
[2] LUO A, PEKGULERYUZ M O. Cast magnesium alloys for elevated temperature applications[J]. J Mater Sci, 1994, 29(20): 5259-5271.
[3] MORDIKE B L. Development of highly creep resistant magnesium alloys[J]. J Mater Process Technol, 2001, 117: 391-394
[4] H I, HRYN J, CLOW B. Magnesium technology 2000[M]. The Minerals Metals & Materials Society, 2000.
[5] JIA Shu-zhuo, XU Chun-jie, ZHANg Zhong-ming, SONG Pei-wei, GUO Xue-feng, MA Sheng-qiang. Microstructures and properties of Mg-9Al-6Si magnesium alloys[J]. Foundry Technology, 2005, 26(12): 1104-1105, 1108.
[6] Yichuan P, Xiangfa L, Hua Yang. Microstructural formation in a hypereutectic Mg-Si alloy[J]. Materials Characterization, 2005, 55(3): 241-247.
[7] JIANG Q C, WANG H Y, WANG Y, MA B X, WANG J G. Modification of Mg2Si in Mg-Si alloys with yttrium[J]. Mater Sci Engin A, 2005, 392: 130-135.
[8] KONG C Y, SOAR R C, DICKENS P M. Ultrasonic consolidation for embedding SMA fibres within aluminium matrices[J]. Composite Structures, 2004, 66(1/4): 421-427.
[9] TSUNEKAWA Y, SUZUKIB H, GENMA Y. Application of ultrasonic vibration to in situ MMC process by electromagnetic melt stirring[J]. Materials and Design, 2001, 22: 467-472.
[10] PAN Lei, TAO Jie, CHEN Zhao-feng, LIU Zi-li. Acoustic effect of high intensity ultrasonic in particles/metal melt[J]. Journal of Materials Engineering, 2006, 1: 35-37. (in Chinese)
[11] AMBRAOV O V. Ultrasound in liquid and solid metals[M]. Boca Raton, F L: CRC Press, 1994.
[12] TAKAMICHI I, RODERICK I L G. The physical properties of liquid metal[M]. XIAN Ai-ping, WANG Lian-wen. Beijing: Science Press, 2005: 142.
Corresponding author: LI Zi-quan; Tel: +86-25-84892797; E-mail: ziquanli@nuaa.edu.cn
(Edited by CHEN Ai-hua)


