
Effects of cooling rates on microstructure and
microhardness of lead-free Sn-3.5%Ag solders
SHEN Jun(沈 骏), LIU Yong-chang(刘永长), HAN Ya-jing(韩雅静), GAO Hou-xiu(高后秀),
WEI Chen(韦 晨), YANG Yu-qin(杨渝钦)
(College of Materials Science and Engineering, Tianjin University, Tianjin 300072, China)
Received 8 May 2005; accepted 22 September 2005
___________________________________________________________________________________________________________________________________________________________________
Abstract:
The microstructure and microhardness of Sn-3.5%Ag solders were explored in the cooling rate ranging from 0.08 to 104 K/s. Under rapid cooling condition, the strong kinetic undercooling effect leads to the actual solidification process starting at the temperature lower than the equilibrium eutectic point, and the actual metastable eutectic point shifts to the higher Ag concentration. Hence, the higher the applied cooling rate is, the more the volume fraction of primary β-Sn crystal forms. At the same time, the separation of primary β-Sn crystal favors restraining the formation of bulk Ag3Sn intermetallic compounds (IMCs) in solder due to the mismatch crystalline orientation relationship, those Ag3Sn phase separating through the eutectic reaction could hardly cling to the primary β-Sn crystal and grow up. Additionally, the Vickers hardness test shows that fine β-Sn and spherical Ag3Sn phase in the rapidly solidified alloy strongly improves the microhardness of the Sn-3.5%Ag solder. Keywords: lead-free solder; Sn-3.5%Ag solder; eutectic reaction; intermetallic compounds; microhardness
___________________________________________________________________________________________________________________________________________________________________
1 Introduction
Increasing considerations on the environmental protection and health hazards of Sn-Pb solders used in electronic packaging have prompted the development for lead-free solder alternatives in electronic industry[1–3]. And the original impetus of lead-free packaging driven by legislation, recently turns into the force such as commercial advantages related to environment-friendly electronics that have raised more interest in industry and speeded the process. In recent years, the application of lead-free solder to consumer electronic products becomes reality. And among new generation of lead-free solders, Sn-Ag alloys with higher strength, superior resistance to creep and thermal fatigue compared with eutectic Sn-Pb solder and cost effectiveness compared with other lead-free solder alternatives have been recognized as one of the best choices to replace conventional Sn-Pb eutectic solder[4-6].
The cooling rate of alloys directly affects the microstructure of the Sn-Ag solders, also significantly influences their mechanical behaviors. Hence, the effects of applied cooling rate on microstructure and mechanical behavior have been studied intensively. OCHOA et al[7, 8] found that the applied cooling rate had a significant effect on intermetallic compounds size, morphology and mechanical properties of Sn-Ag alloys. At relatively fast cooling rates (24 K/s), a fine distribution of spherical particles Ag3Sn is observed. And the intermetallic compounds exhibits a rod-like morphology at lower cooling rates (0.08 K/s). KIM et al[4] studied the solidified microstructure of Sn-Ag-Cu alloys at cooling rates from 0.012 to 8.3 K/s, and as a result, different tensile properties have been obtained in these alloys. DUTTA et al[5] explored the impression creep characterization of rapidly-cooled Sn–Ag solders, but the cooling rate is still only about 31 K/s between 553 and 463 K, and 20 K/s between 463 and 333 K at the bottom of the sample. To our knowledge, the relationship between the solidified microstructure of Sn-3.5Ag solder in relative rapid cooling rate (104 K/s), and the resultant microhardness of it is still an open question.
At the same time, it is usually considered that increasing the applied cooling rate of eutectic Sn-Ag solder or using hypoeutectic Sn-Ag solder would avoid the formation of bulk Ag3Sn IMCs in the solders and ascribe this to that the primary Ag3Sn crystal nucleates and grows need minimal undercooling but the β-Sn phase requires significantly larger undercooling to nucleate and solidify finally. But no one explains whether the primary Ag3Sn crystal corresponds to the bulk needle-like Ag3Sn IMCs formed in solder.
Here a Sn-3.5%Ag eutectic alloy was used as an example for investigating the influence of cooling rates on the microstructure of solders and the inherent mechanism of the formation of fine primary β-Sn crystals and the bulk Ag3Sn IMCs. To determine the microhardness of the solders solidified with different cooling rates, Vickers hardness measurement was performed on four samples.
2 Experimental
2.1 Sample preparation
Sn-3.5%Ag solders (in mass) were prepared from bulk rods of pure Sn, Ag in eutectic composition. The process of melting was carried out in a vacuum arc furnace under a high purity argon atmosphere to produce button-like specimen with a diameter of approximately 3.5 cm. In order to get a homogeneous composition within the ingots, all alloys were remelted four times.
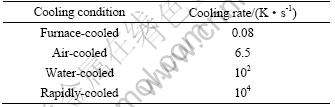
After that, the melts were cooled down to ambient temperature at four different cooling rates obtained by changing the cooling media as furnace, air and water. The highest cooling rate is achieved by casting alloy melt into a copper mold to obtain a rod with a diameter of 5 mm. Table 1 lists the applied cooling rates of the four samples.
Table 1 Applied cooling rates in different media
2.2 Microstructural observation
The Sn-3.5%Ag specimens were prepared by standard metallographic procedures and the specimens were etched with a solution of 5%(volume fraction, the same below) HNO3+95%C2H5OH. Optical microscopy and scanning electron microscopy were used for the observation of microstructures. X-ray diffraction was adopted for the determination of phase structure in the solders.
2.3 Hardness measurement
Vickers hardness measurement was performed to clarify the relationship of microstructure and microhardness. The corresponding Vickers hardness values were obtained as[9]
![]() (1)
(1)
where φ is the indenter apex angle, F is the applied load and d is the average length of diagonals. Here the applied load and loading period are 0.1 N and 5 s, respectively. For each sample, twenty-five points were tested and the arithmetical mean values were obtained.
3 Results and discussion
3.1 Effect of cooling rate on microstructure
Microstructure observation shows that the furnace-cooled sample, with a cooling rate of 0.08 K/s, nearly follows the equilibrium solidification process, so exhibits a full eutectic structure consisting of gray and white phases(Fig.1(a)). According to the corresponding X-ray diffraction spectrum (Fig.2) of this sample, it can be seen that the eutectic structure consists of a mixture of intermetallic compounds Ag3Sn and β-Sn phase and the eutectic reaction is L→Ag3Sn+β-Sn.
With increasing cooling rate, different morphologies appear for the specimens because of the non-equilibrium solidification conditions. Contrary to the fine eutectic structure in the furnace-cooled sample, these samples show clear dendritic structures. Typical microstructures of those specimens exhibit two kinds of white and black areas. According to the X-ray diffraction spectrum in Fig.2, it is shown that large white dendrites are primary β-Sn phase surrounded by a net-like eutectic mixture of β-Sn phase and Ag3Sn.
To determine the volume fraction of primary β-Sn dendrites for the air-cooled, water-cooled and rapidly- cooled Sn-3.5%Ag solders, an area analysis way[10] was used and the result is listed in Table 2. It is found out that the volume fraction of β-Sn dendrites of the samples increases gradually with increasing cooling rate.
In order to accurately determine the influence of applied cooling rate on the morphology of primary β-Sn dendrites, linear intercept method[11] was employed to determine the secondary dendrite arm spacing (SDAS) values between the lamellae in the observed microstructures. Twenty locations were chosen randomly in the samples and the arithmetical mean
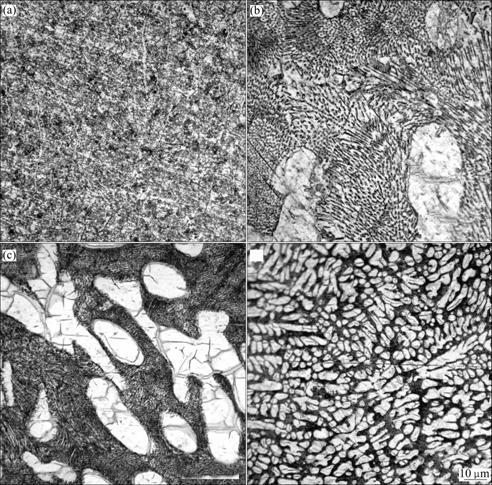
Fig.1 Optical photographs of Sn-3.5%Ag solders solidified under different cooling conditions: (a) Furnace-cooled; (b) Air-cooled; (c) Water-cooled; (d) Rapidly-cooled
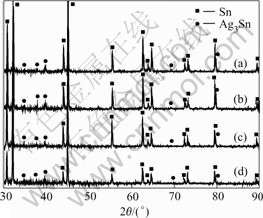
Fig.2 X-ray diffraction spectrum of Sn-3.5%Ag solders: (a) Rapidly-cooled; (b) Water-cooled; (c) Air-cooled; (d) Furnace-cooled
values were considered the results. After that, the variation of SDAS in the solidified Sn-3.5%Ag solders is presented as a function of local solidification time (Table 3). It is found that the measured SDAS data were
Table 2 Measured volume fractions of primary β-Sn dendrites in Sn-3.5%Ag solders

Table 3 Comparison of secondary dendrite arm spacing of primary β–Sn dendrites in Sn-3.5%Ag solders

adopted to fit to the following equation[11].
![]() (2)
(2)
where d is the secondary dendrite arm spacing, tf is the local solidification time, a and n are parameters depending on the material. In our study, for Sn-3.5%Ag eutectic alloy, the determined parameters a and n equal 3.7 and 0.43, respectively.
The influences of applied cooling rates on the microstructure of solidified Sn-3.5%Ag solders could be mainly attributed to the different solidification routes. Fig.3 illustrates the corresponding solidification routes of Sn-3.5%Ag solders under different conditions. During equilibrium solidification, the investigated Sn-3.5%Ag alloy experiences the eutectic reaction, L→Ag3Sn+β-Sn at Te (the eutectic point marked as o in Fig.3). While in the non-equilibrium solidification, degree of kinetic undercooling ΔT was achieved, the metastable eutectic point shifts to point c (Fig.3). Hence, the explored Sn-3.5%Ag alloy results as hypoeutectic compositions, and the solidification processing turns into L→primary β-Sn+L and L→Ag3Sn+β-Sn. Therefore, the higher the applied cooling rate, the higher the concentration of the metastable eutectic points apart from the equilibrium eutectic point o, and the more of the primary β-Sn separates out from the melt. These considerations are consistent with the microstructure observations (Fig.1), X-ray diffraction spectrum (Fig.2) and the volume fraction of β-Sn dendrites (Table 2).
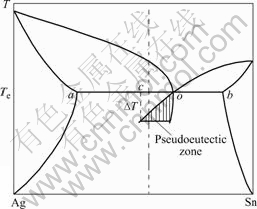
Fig.3 Schematical diagram of Sn-3.5%Ag eutectic alloy solidified in metastable pseudoeutectic zone
Compare with the specimens cooled by three cooling rates, finer dendrite microstructure and higher volume fraction of primary β-Sn crystal form in the rapidly- cooled specimen (Fig.1) because the rapid solidification process restrains the diffusion of solute tin. And the cooling rate also plays a significant role on the morphology of eutectic structure (Fig.4). The high solidification rate obtained by rapid cooling condition promotes nucleation and suppresses the growth of Ag3Sn in eutectic structure, yielding a fine Ag3Sn with spherical morphology.
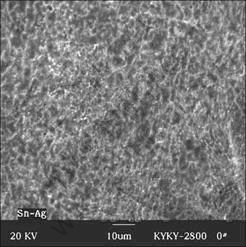
Fig.4 SEM image of typical eutectic structure in rapidly-cooled Sn-3.5%Ag solder
3.2 Formation of bulk Ag3Sn in furnace-cooled sample
Fig.5 shows that there are very few bulk Ag3Sn IMCs formed in the furnace-cooled specimen. This bulk needle-like Ag3Sn IMCs may seriously influence the mechanical performance due to the fact that they may lead to brittle Ag3Sn IMCs, which may lead to serious problems under stressed conditions in the actual service for printed wiring boards[4, 6-8].

Fig.5 Typical microstructure of bulk Ag3Sn IMCs in furnace-cooled Sn-3.5%Ag solder
No bulk Ag3Sn IMCs have been found in air-cooled, water-cooled and rapidly-cooled specimens. This corresponds quite well with other reported results that high cooling speed can significantly restrain the formation of bulk Ag3Sn IMCs in Sn-Ag lead-free solder and bulk Ag3Sn IMCs are inclined to form under relative lower cooling conditions[4, 6, 7].
Fig.6 shows the influence of different primary crystals, Ag3Sn or β-Sn phase, on the formation and growth of the bulk Ag3Sn IMCs in solders melt. The inherent mechanism that the high cooling rates prevent the separation of bulk Ag3Sn IMCs in the Sn-3.5%Ag solder melt could be explained as follows. For the investigated Sn-3.5%Ag alloy, the involved two phases possess different growth velocities in the eutectic reaction, thus a leading phase Ag3Sn appears in the melt in eutectic reaction[12]. Hence, if a primary Ag3Sn crystal formed before the onset of eutectic reaction, the eutectic Ag3Sn phase can nucleate around the primary Ag3Sn crystal and grow into the surrounding melt due to the matching crystalline structures[13]. This would lead to the formation of bulk Ag3Sn IMCs easily. But when β-Sn phase acts as the primary crystal, it is hard for the Ag3Sn separating in eutectic reaction to cling around the primary β-Sn crystal due to the mismatch crystalline orientation relationships (Ag3Sn has a facet structure but β-Sn a non-facet structure), the Ag3Sn has to nucleate by themselves. Hence, it is hard to form bulk Ag3Sn IMCs through eutectic reaction when the primary crystal is β-Sn phase.
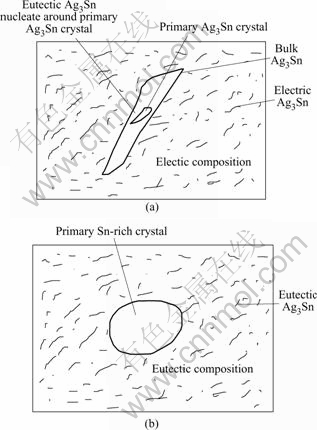
Fig.6 Schematical diagram of formation of bulk Ag3Sn IMCs with primary crystal of Ag3Sn(a) and β-Sn phase(b)
Note that the metastable eutectic point shifts to the direction of higher Ag concentration point c (Fig.3) under rapid solidification conditions. So primary β-Sn crystal separates out before the onset of eutectic reaction and restrains the formation of bulk Ag3Sn IMCs in the solders. But in slowly-cooled solders, there may be some Ag-rich zone forming in the melt before the onset of eutectic reaction which can lead to the formation of bulk Ag3Sn IMCs. This would largely explain why the rapid cooling rate could restrain the separation of bulk Ag3Sn IMCs in Sn-3.5%Ag eutectic alloy[4, 6].
3.3 Effect of cooling rate on microhardness
The measurement of microhardness is normally adopted to characterize the mechanical properties of alloys. Because the test can be performed in very small zone, and can determine the hardness of individual grain, phases, and structural component of alloy. During the measurement, the loading force and time must be chosen to fit the test. In ISLAM et al’s study[14], 2 N and 15 s was used to determine the microhardness of Sn-Zn, Sn-Zn-Bi and Sn-Pb alloys. BHAHAY et al[15] studied the hot microhardness of Sn-3.5%Ag alloy with 0.5 N and 5 s.
Fig.7 illustrates the measured data for Vickers hardness in four Sn-3.5%Ag specimens. The microhardness of whole Sn-3.5%Ag solders increased obviously with increasing cooling rate except the water-cooled sample is slightly lower than the air-cooled sample. To clarify this, the Vickers hardness of eutectic zone in air-cooled and water-cooled Sn-3.5%Ag solders was performed. (Here, because the dendrite of rapidly-cooled sample is very fine and the eutectic zone in solder is really small, the test of eutectic zone in rapidly-cooled sample cannot be performed even using the smallest load). It is found out that increasing the cooling rate can strongly improve the hardness of eutectic zone in Sn-3.5%Ag solders.

Fig.7 Measured Vickers hardness of Sn-3.5%Ag solders, eutectic zone of Sn-3.5%Ag solders solidified at different cooling rates
As reported before[16], the hardness of Ag3Sn is about 15 times larger than that of pure Sn and 12 times larger than that of eutectic structure in Sn-3.5%Ag solder. In our study, the hardness of bulk Ag3Sn formed in furnace-cooled specimen is about 67 HV, which is about three or four times larger than that of the investigated solders solidified under different cooling conditions. So it might be concluded that Ag3Sn IMCs acts as a reinforcing phase in solder and thus the corresponding amount and distribution of Ag3Sn would largely influence the microhardness.
This could explain why increasing the cooling rate, the Sn-3.5%Ag samples exhibits higher microhardness. With increasing cooling rate, fine β-Sn dendrites formed in the sample and spherical like Ag3Sn formed in eutectic zone, those can benefit improving the surface area per unit volume and can limit the dislocation lines pass through the solder. Hence, the sample becomes harder. But for the air-cooled and water-cooled Sn-3.5%Ag samples, the microhardness is also influenced by the soft bulk β-Sn dendrites. So, the whole microhardness of two solders became similar.
4 Conclusions
1) Rapid solidification prompts the formation of a metastable pseudoeutectic region. So the higher the applied cooling rates is, the higher volume fraction of primary β-Sn crystal forms.
2) The obtained secondary dendrite arm spacing in Sn-3.5%Ag eutectic alloys decreases gradually with increasing applied cooling rates, and fits will with the equation is ![]() .
.
3) The separation of primary β-Sn crystal restrains the formation of bulk needle-like Ag3Sn in the rapidly solidified solders. On the contrary, primary Ag3Sn crystal is beneficial to forming bulk needle-like Ag3Sn IMCs in the slowly-cooled solder.
4) The hardness of Sn-3.5%Ag alloy is controlled by the tough Ag3Sn and soft β-Sn phase. So, rapidly-cooled solder with fine β-Sn and spherical Ag3Sn phase in eutectic structure exhibits the highest microhardness.
References
[1] AB TEW M, SELVADURAY G. lead-free solders in microelectronics [J]. Mater Sci Eng R, 2000, R27: 95-141.
[2] ZENG K, TU K N. Six cases of reliability study of Pb-free solder joints in electronic packaging technology [J]. Mater Sci Eng R, 2002, R38: 55-105.
[3] WU C M L, YU D Q, LAW C M T, et al. Properties of lead-free solder alloys with rare earth element additions [J]. Mater Sci Eng R, 2004, R44: 1-44.
[4] KIM K S, HUH S H, SUGANUMA K. Effects of cooling speed on microstructure and tensile properties of Sn-Ag-Cu alloys [J]. Mater Sci Eng A, 2002, A333: 106-114.
[5] DUTTA I, PARK C, CHOI S. Impression creep characterization of rapidly cooled Sn-3.5Ag solders [J]. Mater Sci Eng A, 2004, A379: 401-410.
[6] KIM K S, HUH S H, SUGANUMA K. Effects of intermetallic compounds on properties of Sn-Ag-Cu lead-free soldered joints [J]. J Alloys Comp, 2003, 352: 226-236.
[7] OCHOA F, WILLIAMS J J, CHAWLA N. Effect of cooling rate on the microstructure and mechanical behavior of Sn-3.5Ag solder [J]. J O M 2003, 55: 56-60.
[8] OCHOA F, WILLIAMS J J, CHAWLA N. Effects of cooling rate on the microstructure and tensile behavior of a Sn-3.5wt% Ag solder [J]. J Electron Mater, 2003, 32: 1414-1420.
[9] KURODA M, SETOYAMA D, UNO M, et al. Nanoindentation studies of zirconium hydride[J]. J Alloys Com, 2004, 368: 211-214.
[10] VANDER-VOORT G F. Metallography Principles and Practice[M]. New York: McGraw-Hill, 1984.67-68.
[11] FLEMINGS M C. Solidification Processing [M]. New York, McGraw-Hill, 1974.6-7.
[12] HENDERSON D W, GOSSELIN T, SARKHEL A. Ag3Sn plate formation in the solidification of near ternary entectic Sn-Ag-Cu alloys [J]. J Mater Res, 2002, 17(11): 2755-2778.
[13] CHALMERS B. Principles of Solidification [M]. New York: John Wiley & Sons Inc, 1964. 70-71.
[14] ISLAM R A, WU BY, ALAM M O, et al. Investigations on microhardness of Sn-Zn based lead-free solder alloys as replacement of Sn-Pb solder [J]. J Alloys Comp, 2005, 392: 149-158.
[15] BHAHAY M M EL, MOSSALAMY M E EL, MAHDY M, et al. Some mechanical properties of Sn-3.5Ag eutectic alloy at different temperatures [J]. J Mater Sci: Mater in Elec, 2004, 15: 519-526.
[16] DENG X, CHAWLA N, CHAWLA K K, et al. Deformation behavior of (Cu, Ag)-Sn intermetallics by nanoindentation [J]. Acta Mater, 2004, 52: 4291-4303.
___________________
Foundation item: Project(50401033) supported by the National Natural Science Foundation of China; Project(200335) supported by the Foundation for the Author of National Excellent Doctoral Dissertation of China; Project(033608811) supported by the Natural Science Foundation of Tianjin City, China; Project supported by the Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Education Ministry
Corresponding author: SHEN Jun; Tel: +86-22-81982940; Fax: +86-22-27405874; E-mail: shenjun2626@163.com
(Edited by LONG Huai-zhong)


