
Shape-controlled synthesis of nanocubic Co3O4 by hydrothermal oxidation method
YANG You-ping(杨幼平), HUANG Ke-long(黄可龙), LIU Ren-sheng(刘人生),
WANG Li-ping(王丽平), ZENG Wen-wen(曾雯雯), ZHANG Ping-min(张平民)
School of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China
Received 30 December 2006; accepted 9 April 2007
Abstract:
The nanocubic Co3O4 was synthesized by hydrothermal oxidation method. The effects of cobalt salt, precipitating agent, surfactant, solvent, pH value of the suspension and the amount of oxidant H2O2 on the morphology and structure of Co3O4 were investigated. The Co3O4 powders were characterized by transmission electron microscope and X-ray diffraction. The results show that the morphology of Co3O4 is closely dependant on the anion in cobalt salts, but it is not so sensitive to the precipitating agents and solvents. The amount of H2O2 is the key factor to obtain Co3O4 with spinel crystal structure. The optimum synthetic conditions of uniform shape-controlled Co3O4 nanocubes are as follows: Co(CH3COO)2·4H2O as cobalt salt, KOH as precipitating agent, polyethylene glycol with relative molecular mass of about 20 000 as surfactant, water-n-butanol as solvent system, pH value of 8-9, the molar ratio of H2O2 to Co2+ above 2.5:1.0, hydrothermal temperature of 160 ℃ and hydrothermal holding time of 10 h. The tap density and apparent density of nanocubic Co3O4 obtained with the average particle size of 20 nm are 1.01 g/cm3 and 0.70 g/cm3, respectively.
Key words:
Co3O4; nanocubes; shape-controlled; hydrothermal oxidation;
1 Introduction
The tricobalt tetraoxide Co3O4 belongs to the normal spinel crystal structure based on a cubic close packing array of oxide atoms, in which Co2+ ions occupy the tetrahedral 8a sites and Co3+ ions occupy the octahedral 16d sites. In recent years, Co3O4 has attracted attention due to its wide applications in catalysts[1], magnetic semiconductors[2], electrode material[3-5], gas sensors[5] and pressure sensitive ceramics[6]. Various methods, such as the thermal decomposition of solid phase[7-8], sol-gel method[9], hydrothermal method[10-11], solvothermal decomposition[12], chemical vapor deposition[13], liquid-control- precipitation method[14] and spray pyrolysis[15], were attempted to synthesize nanosized spinel Co3O4. It is well-known that the behaviors of nanophase materials strongly depend on the shape and size of the particles[5]. And hydrothermal oxidation method is an efficient technique for preparing fine uniform particles of metal oxides[16].
ZHANG et al[10] studied the effects of hydro- thermal synthetic conditions, such as the starting concentration of Co(NO3)2 solution, pH value, hydrothermal temperature, holding time and the stocking mode, on the shape and size of Co3O4 cubes in Co(NO3)2-NH3·H2O system. The Co3O4 and β-Co(OH)2 mixtures were obtained when the temperature was below 180 ℃ and hydrothermal holding time was 1-36 h, and the cubic Co3O4 could be obtained by calcining the mixtures in air. JIANG et al[11] reported that Co(OH)2 gel, which was prepared using CoSO4·7H2O and NH3·H2O as starting materials, could be oxidized to nanocrystalline Co3O4 by hydrogen peroxide in a hydrothermal system at 180 ℃ for 24 h. Although Co(OH)2 gel was filtered using vacuum filtration and washed by distilled water for several times until no ![]() and
and ![]() remained, the morphology of nanocrystalline Co3O4 was irregular. In order to synthesize uniform shape-controlled Co3O4 nanocubes, the effects of anion in cobalt salt, precipitating agent, surfactant, solvent, pH value of the suspension, and the amount of oxidant H2O2 on the morphology and structure of Co3O4 were investigated in this study.
remained, the morphology of nanocrystalline Co3O4 was irregular. In order to synthesize uniform shape-controlled Co3O4 nanocubes, the effects of anion in cobalt salt, precipitating agent, surfactant, solvent, pH value of the suspension, and the amount of oxidant H2O2 on the morphology and structure of Co3O4 were investigated in this study.
2 Experimental
15 mmoL cobalt salt was dissolved into distilled water containing certain surfactant and organic solvent, and then excessive amount of precipitating agent was added with electromagnetic stirring at 30 ℃ during the formation of Co(OH)2 precursor. The pH value of the suspension after precipitation reaction was monitored to 8-9. A certain amount of 30% (mass fraction) H2O2 was dropped into the suspension. Finally, all of them were transferred into a Teflon-lined stainless steel autoclave with the volume of 100 mL, and the autoclave was filled with distilled water up to 70% of the total capacity. The sealed autoclave was heated to 160 ℃ and maintained for 10 h, then cooled to room temperature in air naturally. The black powders were centrifuged and washed with distilled water and absolute ethanol for three times, respectively, and dried in an oven at 80 ℃ for 6 h.
The morphology and size of the obtained powders were determined by using a Japan JEOL JEM-1230 transmission electron microscopy(TEM). The X-ray diffraction(XRD) patterns of the powders were obtained with a Japan Rigaku D/max-2500 X-ray diffractometer using Cu Kα radiation in the 2θ range from 10? to 80?.
3 Results and discussion
3.1 Effect of cobalt salt and precipitating agent
Fig.1 shows the TEM images of Co3O4 using different cobalt salts and precipitating agents in the presence of polyethylene glycol with relative molecular mass of about 20 000(PEG 20 000) and water-n-butanol solvent system. It can be seen from Figs.1(a) and (d) that the morphologies of Co3O4 are irregular nanocubes using Co(NO3)2·6H2O as cobalt salt and KOH or NH3-NH4Cl buffer solution as precipitating agent. For comparison typical spherical Co3O4 and nanocubic Co3O4 are obtained when the cobalt salts are CoSO4·7H2O and Co(CH3COO)2·4H2O, respectively, as shown in Figs.1(b) and (c). It can be concluded that the morphologies of Co3O4 are closely dependant on the anion type in cobalt salts. In other words, the anion type in cobalt salt plays a KOH; (c) Co(CH3COO)2 and KOH; (d) Co(NO3)2 and NH3-NH4Cl buffer solution key role in the morphology of Co3O4, while the influence of precipitating agent on the morphology of Co3O4 is very limited. Therefore, Co(CH3COO)2·4H2O and KOH are chosen as cobalt salt and precipitating agent to synthesize nanocubic Co3O4, respectively.
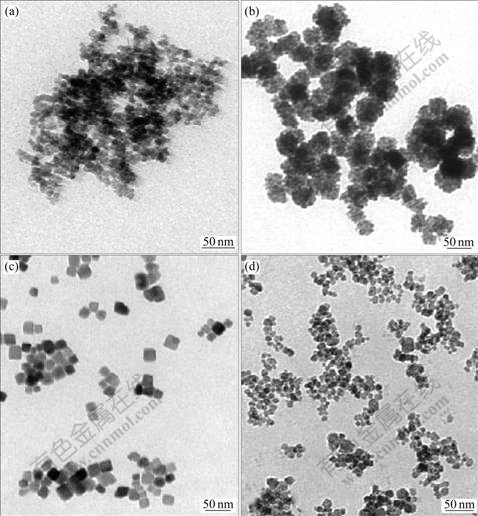
Fig.1 TEM images of Co3O4 synthesized with different cobalt salts and precipitating agents: (a) Co(NO3)2 and KOH; (b) CoSO4 and
3.2 Effect of surfactant
Fig.2 shows the effects of surfactant on morphology of Co3O4 in water-n-butanol solvent system. It can be seen that nanocubic Co3O4 synthesized in the presence of non-ionic surfactant PEG 20 000 is highly dispersed and shows excellent uniformity, while Co3O4 nanoparticles obtained from anionic surfactant sodium dodecyl benzene sulfonate(SDBS) are agglomerated in irregular shapes. This may be due to the interface retarding effect of PEG 20 000. The relative molecular mass of PEG 20 000 is greater than that of SDBS. As a result, PEG 20 000 is chosen as the surfactant in the synthesis of nanocubic Co3O4.
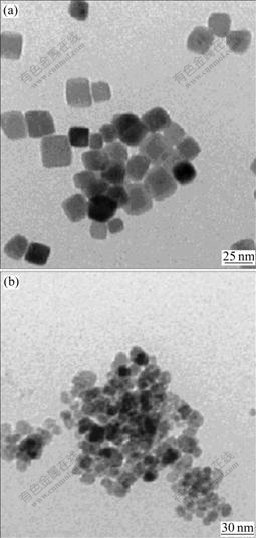
Fig.2 TEM images of Co3O4 synthesized with different surfactants: (a) Polyethylene glycol 20 000; (b) Sodium dodecyl benzene sulfonate
3.3 Effect of pH value
Fig.3 shows the TEM image of Co3O4 synthesized in suspension of pH 11-12 after precipitation reaction. Compared Fig.1(c) with Fig.3, it is very obvious that nanocubic Co3O4 with the average particle size of 20 nm is formed when pH is 8-9, and when pH goes up to 11-12 irregular Co3O4 including some grains recombined in the products becomes serious. Because the condensation reaction of Co(OH)2 precursor can easily occur at higher pH value, agglomeration of the nanoparticles occurs.
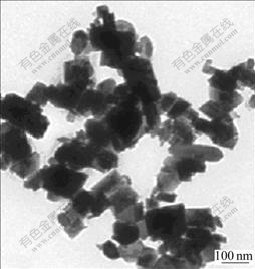
Fig.3 TEM image of Co3O4 synthesized in suspension of pH 11-12
The condensation reaction of Co(OH)2 can be expressed as
—Co—O—H+H—O—Co—→—Co—O—Co—+H2O (1)
In order to synthesize nanocubic Co3O4, the pH value of suspension should be strictly controlled at 8-9.
3.4 Effect of solvent
The XRD patterns of Co3O4 synthesized in different solvent systems are shown in Fig.4. All peaks shown in Fig.4 can be indexed to a cubic spinel crystal structure Co3O4. No impurity peaks are observed, which indicates that the final product synthesized is Co3O4 with spinel crystal structure under hydrothermal oxidation condition. Based on Scherrer formula, the average particle sizes of Co3O4 in water, water-alcohol and water-n-butanol solvent systems are calculated to be 27 nm, 10 nm and 15 nm, respectively.
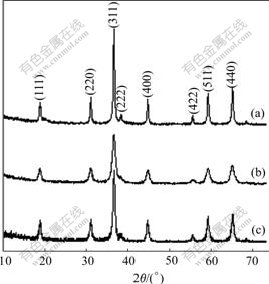
Fig.4 XRD patterns of Co3O4 synthesized in different solvent systems: (a) Water; (b) Water–alcohol; (c) Water-n-butanol
Fig.5 shows the TEM images of Co3O4 synthesized in water and water-alcohol solvent systems using Co(CH3COO)2·4H2O as cobalt salt. Compared Fig.1(c) with Fig.5, it can be seen that nanocubic Co3O4 particles are all obtained in these solvent systems. While Co3O4 synthesized in water-n-butanol solvent system shows a better monodisperse sign (Fig.1(c)), and the tap density and apparent density of uniform shape-controlled Co3O4 nanocubes are 1.01 g/cm3 and 0.70 g/cm3, respectively.

Fig.5 TEM images of Co3O4 synthesized in different solvent systems: (a) Water; (b) Water-alcohol
3.5 Effect of amount of H2O2
In order to get Co3O4 with spinel crystal structure, the amount of oxidant H2O2 should be enough. The chemical reaction in the hydrothermal oxidation process can be expressed as
3Co(OH)2+H2O2→Co3O4+4H2O (2)
So the molar ratio of H2O2 to Co(OH)2 is 1?3 in theory. However, H2O2 tends to decompose in the practical operation, therefore the amount of H2O2 is far more than the theoretical value.
Fig.6 shows the XRD patterns of the samples obtained with adding different amount of H2O2. When the molar ratio of H2O2 to Co2+ is 2.0?1.0, the impurity Co(OH)2 still exists. While the molar ratio of H2O2 to Co2+ is increased to 2.5?1.0, Co3O4 with cubic spinel crystal structure is obtained. So in order to obtain Co3O4 with spinel crystal structure, the molar ratio of H2O2 to Co2+ should be higher than 2.5?1.0.
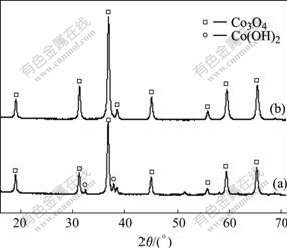
Fig.6 XRD patterns of Co3O4 synthesized with different molar ratios of H2O2 to Co2+: (a) 2.0?1.0; (b) 2.5?1.0
4 Conclusions
1) The uniform shape-controlled spinel Co3O4 nanocube is prepared by hydrothermal oxidation method. The optimum synthetic conditions of Co3O4 nanocubes are as follows: Co(CH3COO)2·4H2O as cobalt salt, KOH as precipitating agent, polyethylene glycol 20 000 as surfactant, pH value of 8-9, molar ratio of H2O2 to Co2+ above 2.5?1.0, hydrothermal temperature of 160 ℃ and hydrothermal holding time of 10 h.
2) The morphology of Co3O4 is closely dependant on the anion in cobalt salts. The nanocrystalline, spherical and uniform nanocubic Co3O4 particles are obtained using Co(NO3)2·6H2O, CoSO4·7H2O and Co(CH3COO)2·4H2O as cobalt salts, respectively.
3) The precipitating agent and solvent system have little influence on morphology of Co3O4. The Co3O4 nanocubes are all synthesized in water, water-alcohol and water-n-butanol solvent systems, and the average particle sizes of Co3O4 are calculated to be 27, 10 and 15 nm, respectively. The tap density and apparent density of uniform shape-controlled Co3O4 nanocubes synthesized in water-n-butanol solvent system are 1.01 g/cm3 and 0.70 g/cm3, respectively.
4) The amount of H2O2 is the key factor to obtain Co3O4 with spinel crystal structure. The molar ratio of H2O2 to Co2+ should be higher than 2.5?1.0.
References
[1] WANG Chen-bin, TANG Chih-wei, GAU Shiue-Jiun, CHIEN Shu-hua. Effect of the surface area of cobaltic oxide on carbon monoxide oxidation [J]. Catalysis Letters, 2005, 101(1/2): 59-63.
[2] ICHIYANAGI Y, KIMISHIMA Y, YAMADA S. Magnetic study on Co3O4 nanoparticles [J]. Journal of Magnetism and Magnetic Materials, 2004, 272: e1245-e1246.
[3] WANG G X, CHEN Y, KONSTANTINOV K, LINDSAY M, LIU H K, DOU S X. Investigation of cobalt oxides as anode materials for Li-ion batteries [J]. Journal of Power Sources, 2002, 109: 142-147.
[4] YUAN Zheng-yong, HUANG Feng, FENG Chuan-qi, SUN Ju-tang, ZHOU Yun-hong. Synthesis and electrochemical performance of nanosized Co3O4 [J]. Materials Chemistry and Physics, 2003, 79: 1-4.
[5] LI Wei-yang, XU Li-na, CHEN Jun. Co3O4 nanomaterials in lithium-ion batteries and gas sensors [J]. Advanced Functional Materials, 2005, 15(5): 851-857.
[6] CAO Quan-xi, ZHOU Xiao-hua, CAI Shi-dong. The function and effect of Co3O4 on suppress sensitive ceramics [J]. Piezoelectrics and Acoustooptics, 1996, 18(4): 260-263. (in Chinese)
[7] ARDIZZONE S, SPINOLO G, TRASATTI S. The point of zero charge of Co3O4 prepared by thermal decomposition of basic cobalt carbonate [J]. Electrochimca Acta, 1995, 40(16): 2683-2686.
[8] LIAO Chun-fa, LIANG Yong, CHEN Hui-huang. Preparation and characterization of Co3O4 by thermal decomposition from cobalt oxalate [J]. The Chinese Journal of Nonferrous Metals, 2004, 14(12): 2131-2136. (in Chinese)
[9] CAO Jin-zhang, ZHAO Yan-chun, YANG Wu, TIAN Jian-niao, GUAN Fei, MA Yong-jun. Sol-gel preparation and characterization of Co3O4 nanocrystals [J]. Journal of University of Science and Technology Beijing, 2003, 10(1): 54-57.
[10] ZHANG Wei-min, SONG Xin-yu, LI Da-zhi, YU Hai-yun, SUN Si-xiu. Effects of hydrothermal synthetic conditions on Co3O4 cubes morphologies [J]. Chemical Journal of Chinese University, 2004, 25(5): 797-801. (in Chinese)
[11] JIANG Yang, WU Yue, XIE Bo, XIE Yi, QIAN Yi-tai. Moderate temperature synthesis of nanocrystalline Co3O4 via gel hydrothermal oxidation [J]. Materials Chemistry and Physics, 2002, 74: 234-237.
[12] NETHRAVATHI C, SEN S, RAVISHANKAR N, RAJAMATHI M, PIETZONKA C, HARBRECHT B. Ferrimagnetic nanogranular Co3O4 through solvothermal decomposition of colloidally dispersed monolayers of α-cobalt hydroxide [J]. J Phys Chem B, 2005, 109(23): 11468-11472.
[13] BAHLAWANE N, FISCHER RIVERA E, KOHSE-HOINGHAUS K, BRECHLING A, KLEINEBERG U. Characterization and tests of planar Co3O4 model catalysts prepared by chemical vapor deposition [J]. Applied Catalysis B: Environmental, 2004, 53: 245-255.
[14] LI Ya-dong, HE Yun-pu, LI Long-quan, QIAN Yi-tai. Fabrication of Co3O4 ultrafines by a liquid-control-precipitation method [J]. Chemical Journal of Chinese University, 1999, 20(4): 519-522. (in Chinese)
[15] SHINDE V R, MAHADIK S B, GUJAR T P, LOKHANDE C D. Supercapacitive cobalt oxide (Co3O4) thin films by spray pyrolysis [J]. Applied Surface Science, 2006, 252: 7487-7492.
[16] XU Ru-ren, PANG Wen-qin. Inorganic synthesis and preparative chemistry [M]. Beijing: Higher Education Press, 2001. (in Chinese)
Foundation item: Project(50542004) supported by the National Natural Science Foundation of China; Project(ZE097) supported by Creative Program of Central South University, China
Corresponding author: HUANG Ke-long; Tel: +86-731-8879850; E-mail: klhuang@mail.csu.edu.cn


