J. Cent. South Univ. Technol. (2007)02-0202-04
DOI: 10.1007/s11771-007-0040-1 ![]()
Adsorption property of citrate dispersant on BaTiO3 particles in
aqueous solution
SU Tao-long(苏滔珑), ZHUANG Zhi-qiang(庄志强)
(School of Materials Science and Engineering, South China University of Technology, Guangzhou 510640, China)
Abstract:
Dispersion behavior of ultra fine BaTiO3 particles in the aqueous solution of ammonium citrate (NH4-CA) or citric acid lanthanum chelate (NH4-La-CA) was investigated. The dispersion property was characterized with sedimentation value. It is easier to obtain well dispersed slurry with NH4La-CA than NH4-CA. In an attempt to better understand the role of citric acid radical, simulation of the dispersant adsorption on BaTiO3 particle was performed with universal force field (UFF). It is demonstrated that the interaction between citric acid radical and BaTiO3 particle surface is a weak chemical adsorption. Trivalent citric acid radical is adsorbed on BaTiO3 particle surface with maximal adsorption energy. And, larger molecules of NH4-La-CA formed by adding La3+ lead to better dispersion property than NH4-CA.
Key words:
citrate dispersant; BaTiO3; adsorption ;
1 Introduction
Barium titanate (BaTiO3) has been widely used in multilayer ceramic capacitors (MLCCs), thermistors, piezoelectric and humidity sensor. Well-dispersed slurry is required to produce green sheet with high packing densities and uniform microstructures. BaTiO3 powders can be well-dispersed in many organic solvents, such as acetone, toluene and n-hexane. However, the use of aqueous media is more desirable due to economic and environmental considerations. Recently, the BaTiO3- water dispersion system has been extensively researched[1-2]. However, so far, there are few literatures concerning adsorption of citrate dispersant on BaTiO3 particles. In this work, study of mechanism of the dispersion of ultra fine BaTiO3 powders with citrate as dispersant was carried out.
2 Experimental
Carboxylates (such as citrate and polyacrylate) were usually used as dispersants for ceramic slurry[3-4]. In this work, ammoniumized citrate (NH4-CA) and citric acid lanthanum chelate (NH4-La-CA) were utilized to disperse ultra fine BaTiO3 powders in water. NH4-La-CA solution was obtained by mixing lanthanum nitrate and ammoniumized citrate solution at pH value of 10. The molar ratio of La to citric acid radical was 0.25. Hydrothermal BaTiO3 (BT) powders with particle size of 0.4 μm and specific surface area of 13 m2/g were used. BT slurry was prepared by dispersing 5 g of the powders into 150 mL distilled water. Ammoniumized citric acid (NH4-CA) or citric acid lanthanum chelate (NH4-La-CA) was added, followed by high-energy ultrasonic dispersing for 10 min. The pH of slurry was adjusted by diluted ammonia and hydrochloric acid. After 40 min, slurry was treated with ultrasonic dispersing for 5 min again, and a sedimentation electronic balance was used to determine the sedimentation volume at 30 ℃. Two kinds of the colloidal with citric acid lanthanum chelate (NH4-La-CA) as dispersant were stirred for 1 h at 25, 85 ℃, respectively. The BaTiO3 particles in BT slurry were centrifuged to subside. The content of La in supernatant was determined by chemical titration analysis.
3 Computational approach
In order to better understand the interactions of citric acid radical with BaTiO3 surface, adsorption modeling calculation was carried out. The experimentally determined BaTiO3 lattice constant (a=b=c=0.401 77 nm, α=β=γ=90?)[5] was used for the production of the BaTiO3 surfaces. BaTiO3 super-cell (3×3) surface with 5 atoms layers’ thickness was made after the BaTiO3 crystal structure was cleaved along the (100) plane and optimized with universal force field (UFF). Subsequently,an amorphous cell containing a citric acid radical was built. The BaTiO3 surface and the amorphous cell were placed in a box to form the initial structure with a 2 nm of vacuum region on citric acid radical amorphous cell (Fig.1(a)).
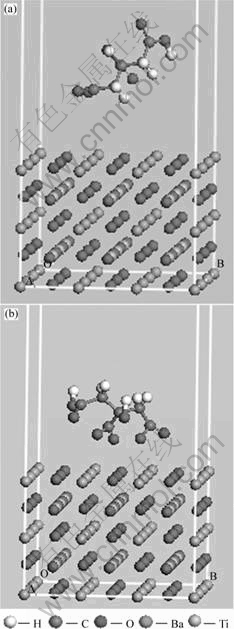
Fig.1 Models before(a) and after (b) adsorption
In this study, universal force field (UFF) was used to simulate the absorption property of citric acid radical on the surface of cubic BaTiO3 particles. UFF developed by RAPP? et al[6] has been parametrized for the full Elements Periodic Table. The set of fundamental parameters is based only on the element, its hybridization and connectivity.
The calculations of universal force field dynamics were performed by using Forcite program package. The number of dynamics steps specified was 105. The target temperature for the simulation and the time for each dynamics step were set as 25 ℃,1 fs,respectively. The NVT thermodynamic ensemble was specified. After dynamics calculation, all of the structures in the trajectory frames were optimized. For optimizing structure calculation, Smart algorithm, Ewald summation method, universal force field and ultra-fine quality of geometry optimization were selected. The convergence tolerance of energy was 8.4×10-5 kJ/mol. After optimization, the lowest energy configuration was selected for adsorption energy calculation.
4 Results and discussion
The dispersion behavior of BaTiO3 powders in water was characterized by sedimentation value derived from the first five minutes. The lower the value is, the better the dispersion is. The relationship between sedimentation value and addition of citric acid radical is shown in Fig.2. Colloid BaTiO3 slurry can be obtained with adding NH4-CA or NH4-La-CA. The conent of NH4-La-CA and NH4-CA for well dispersed slurries is 0.008 2 and 0.007 8, respectively. Fig.2 shows that the same dispersion effect can be obtained with less citric acid lanthanum chelate than ammonium citrate.
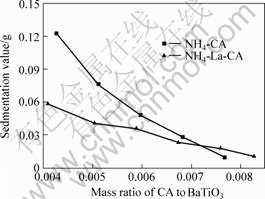
Fig.2 Different dispersion effect between NH4-CA and NH4-La-CA
The dispersive effect of carboxylate on BaTiO3 ultrfine particles results from the structure of dispersant molecule. CARLOS et al[7] investigated the interaction between NH4-PA and BaTiO3 particle by infrared spectroscopy analysis. It is recognized that the NH4-PA molecules can form bidentate chelate or bidentate bridge with Ba atoms on the particle surface during the adsorption of NH4-PA molecules on the surface of BaTiO3 particles. It is evident that oxygen atom of carboxyl in dispersant molecule has an important role in the interaction with BaTiO3 particle surface.
In this study, two kinds of the same colloidal with NH4-La-CA as dispersant were stirred at 25, 85 ℃ for 1 h, respectively. The BaTiO3 particles are centrifuged to subside. No lanthanum is detected in the both kinds of purified supernatant solution. If there are some La in the supernatan solution, it is mainly a physical adsorption between dispersant molecule and BaTiO3 surface in accordance with adsorption characteristics[8]. However, it can be seen from the experiments that there is no La left in solution, that is to say, no dispersant desorption occurred from particle surfaces at higher temperature. Therefore it can be concluded that the adsorption of the dispersant molecule on the surface of BaTiO3 particles is mainly the chemical adsorption.
After the calculation of dynamics equilibrium, the optimized structure is shown in Fig.1(b). The citric acid radical is close to the BaTiO3 surface. Some oxygen atoms of citric acid radical are oriented to point down onto the surface. Oxygen atoms of ionized carboxyl are closer to the surface than the ones on hydroxyl and unionized carboxyl. It shows that the adsorption of citric dispersant results from the interaction between oxygen atoms of carboxyl and BaTiO3 surface.
The adsorption energy (Eadsorp) was calculated by using Eqn.(1), where Etotal is the energy of the surface with adsorbed citric acid radical, Esurface is the energy of the surface without citric acid radical and ECA is the energy of citric acid radical. The result of energy calculation is shown in Table 1.
Eadsorp=Etotal-(Esurface+ECA) (1)
Table1 Adsorption energy of citric acid radical onto BaTiO3 (100) surface

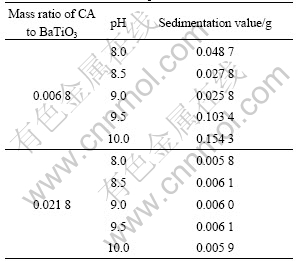
The absolute value of adsorption energy increases with more carboxyl ionized in a citric acid molecule. If all of adsorption energy results from the interaction between the oxygen atom on ionized carboxyl and BaTiO3 surface, the average bonding energy per oxygen atom is about 80 kJ/mol. It is a kind of weak chemical bonding between BaTiO3 and citric acid radical because the chemical adsorption energy is 40-400 kJ/mol[9]. The ionization of citric acid in water depends on pH value. Fig.3 shows the distribution of species in citric acid aqueous solution[10]. Most of citric acid molecules ionize to trivalent ion at pH higher than 8.5 and completely ionize at pH=9. Accordingly, well dispersed slurry can be obtained with NH4-CA as dispersant at pH=8-9. But, pH value higher than 9 will lead to poor dispersion due to coagulation effect of higher concentration of OH- on colloid slurry[11]. Table 2 lists the effect of pH value on the dispersion property of BaTiO3 particles when the mass ratio of CA to BaTiO3 is 0.0068 or 0.021 8. The best dispersion effect of slurry is obtained at pH=9 as the mass ratio of CA to BaTiO3 is 0.006 8. But the dispersion of BaTiO3 particles slurry with proper excessive NH4-CA added is not easily affected by variation of pH value because of the high concentration of ionized citric acid radical.
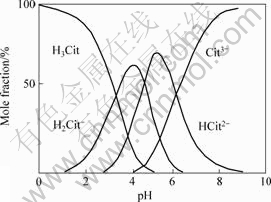
Fig.3 Distribution of species in citric acid aqueous solution[10]
Table 2 Effect of pH value on dispersion property of BaTiO3 particles
The carboxylate groups of the citrate are coordinated to La3+ in monodentate, bidentate and bridging way in citric acid lanthanum chelate[12]. The citric acid radical is as a bridge linking between La3+ and BaTiO3 surface in slurry with citric acid lanthanum chelate as dispersant because the carboxylate groups of the citrate interact not only with La3+ but also with particle surface (Fig.4). The larger molecules of NH4-La-CA are formed by La3+ added in NH4-CA solution. Better dispersion effect is obtained with NH4-La-CA than NH4-CA, because of more strong steric hindrance between particles due to larger molecule.

Fig.4 Schematic illustration of NH4-La-CA being adsorbed on BaTiO3 surface( CA substitutes citric acid radical)
5 Conclusions
1) The adsorption of citric dispersant results from the interaction between oxygen atoms of ionized carboxyl and BaTiO3 surface, and it is a chemical adsorption. The adsorption energy varies with the species of citric acid radical which depends on pH in aqueous solution. Trivalent citric acid radical is adsorbed on BaTiO3 surface with maximal binding energy.
2) The best dispersion effect can be obtained without saturated dispersant at about 9 of pH because citric acid is completely ionized into trivalent citric acid radical on this acidic condition. La3+ in citric acid lanthanum chelate solution is completely attached on BaTiO3 surface by bridging of citric acid radical in slurry. Simultaneously,better dispersion effect can be obtained with dispersant of citric acid lanthanum chelate due to larger molecule than ammonium citrate.
References
[1] BLANCO-LOPEZ M C, RAND B, RILEY F L. The properties of aqueous phase suspensions of barium titanate[J]. J Eur Ceram Soc, 1997, 17: 281-287.
[2] WANG X Y, LU S W, LEE B I. Dispersion and aging behavior of BaTiO3 and PZT in water[J]. Materials Research Bulletin, 2000, 35: 2555-2563.
[3] HIDBER P C, GRAULE T J, GAUCKLER L J. Citric acid-A dispersant for aqueous alumina suspensions[J]. J Am Ceram Soc, 1996, 79(7): 1857-1867.
[4] BIGGS S, SCALES P J, LEONG Y K, et al. Effects of citrate adsorption on the interactions between zirconia surfaces[J]. J Chem Soc Faraday Trans, 1995, 91(17): 2921-2928.
[5] GOTOR F J, REAL C, DIANEZ M J, et al. Relationships between the tecture and structure of BaTiO3 and its tetragonal-cubic transition enthalpie[J]. Journal of Solid State Chemistry, 1996, 123: 301-305.
[6] RAPP? A K, CASEWIT C J, COLWELL K S, et al. UFF, a full periodic table force field for molecular mechanics and molecular dynamics simulations[J]. J Am Chem Soc, 1992, 114: 1002410029.
[7] CARLOS G Y, HEBERTO B R, FROYLA?N M. Colloidal processing of BaTiO3 using ammonium polyacrylate as dispersant[J]. Ceramics International, 2000, 26: 609-616.
[8] ZHEN S L. Surface Modification of Powder [M]. Beijing: China Building Materials Press, 2003.(in Chinese)
[9] ZHAO Z G. Colloid and Surface Chemistry[M]. Beijing: Chemical Industry Press, 2003: 9.(in Chinese)
[10] ZHANG D L, GONG S P, ZHOU D X. Speciation in electroless nickel solutions for BaTiO3-based PTCR ceramics and the roles of complexing agents[J]. Electronic Component and Materials, 2000, 19(1): 17-19.(in Chinese)
[11] LANGE F F. Powder processing science and technology for increased reliability[J]. J Am Ceram Soc, 1989, 72: 3-15.
[12] VANHOYLAND G, PAGNAER J, D’HAEN J, et al. Characterization and structural study of lanthanum citrate trihydrate [La(C6H5O7)(H2O)2]·H2O[J]. Journal of Solid State Chemistry, 2005, 178: 166-171.
Foundation item: Project(020951) supported by Natural Science Fundation of Guangdong Province, ChinaReceived date: 2006-06-28; Accepted date: 2006-08-02
Corresponding author: SU Tao-long, PhD; Tel: +86-760-8223020; E-mail: sutaolong@163.com
(Edited by YUAN Sai-qian)
Abstract: Dispersion behavior of ultra fine BaTiO3 particles in the aqueous solution of ammonium citrate (NH4-CA) or citric acid lanthanum chelate (NH4-La-CA) was investigated. The dispersion property was characterized with sedimentation value. It is easier to obtain well dispersed slurry with NH4La-CA than NH4-CA. In an attempt to better understand the role of citric acid radical, simulation of the dispersant adsorption on BaTiO3 particle was performed with universal force field (UFF). It is demonstrated that the interaction between citric acid radical and BaTiO3 particle surface is a weak chemical adsorption. Trivalent citric acid radical is adsorbed on BaTiO3 particle surface with maximal adsorption energy. And, larger molecules of NH4-La-CA formed by adding La3+ lead to better dispersion property than NH4-CA.


