
J. Cent. South Univ. (2018) 25: 550-560
DOI: https://doi.org/10.1007/s11771-018-3760-5
Synthesis of lithium difluoro(oxalate)borate (LiODFB), phase diagram and ions coordination of LiODFB in dimethyl carbonate
ZHOU Hong-ming(周宏明)1, 2, XIAO Kai-wen(肖凯文)1, LI Jian(李荐)1, 2,XIAO De-min(肖德敏)1, JIANG Yi-xiong(蒋逸雄)1
1. School of Materials Science and Engineering, Central South University, Changsha 410083, China;
2. Hunan Zhengyuan Institute for Energy Storage Materials and Devices, Changsha 410083, China
 Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2018
Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2018
Abstract:
A new two-step synthetic method was successfully developed to simplify the recrystallization process of lithium difluoro(oxalate)borate (LiODFB). Meanwhile, the purity of LiODFB as-prepared was determined by NMR, ICP-AES and Karl Fisher measurements, respectively. The as-prepared LiODFB presents a high purity up to 99.95%. Its metal ions and water contents are under good control as well. Besides, its structure information and thermal properties were confirmed by FTIR, Raman and DSC-TGA analyses, respectively. LiODFB exerts fine thermostability and hypo-water-sensitivity and its structure information agrees well with previous literature. Furthermore, a combination of phase diagram and Raman spectroscopy were utilized to study the thermal phase behavior and ions coordination of LiODFB-DMC binary system to optimize the synthesis and recrystallization process. Although there are three types of molecular interaction forms (CIPs, AGG-IIa, AGG-IIIb) in LiODFB-DMC binary system, LiODFB can only be isolated as large single crystal solvate as LiODFB·(DMC)3/2 by slowly cooling subjected to the nucleation kinetics. Therefore, the fundamental information of our work is helpful in accelerating the application of LiODFB in Li-ion secondary batteries.
Key words:
Cite this article as:
ZHOU Hong-ming, XIAO Kai-wen, LI Jian, XIAO De-min, JIANG Yi-xiong. Synthesis of lithium difluoro(oxalate)borate (LiODFB), phase diagram and ions coordination of LiODFB in dimethyl carbonate [J]. Journal of Central South University, 2018, 25(3): 550–560.
DOI:https://dx.doi.org/https://doi.org/10.1007/s11771-018-3760-51 Introduction
The Li-ion battery is one of the most promising energy storage technologies currently available [1–5]. However, with the introduction of high-voltage cathode materials such as LiNi0.5Mn1.5O4, LiMnPO4 and LiCoPO4, commercial LiPF6-based electrolyte hardly meets the requirements of large-scale Li-ion battery for hybrid electric vehicle (HEV) and electric vehicle (EV) application [4]. Firstly, the high operating voltage of high-voltage cathodes (usually above 4.5 V (vs Li/Li+)) exceeds the electrochemical stability window of commercial LiPF6-based electrolytes, which results in the oxidation of electrolytes and subsequent capacity fading of Li-ion battery system during cycling [5–13]. In addition, LiPF6-based electrolyte is very sensitive to moisture, and its hydrolysates, HF acid, can bring great potential safety troubles to Li-ion batteries [12]. Therefore, the exploration of new electrolyte is urgently required to advance new Li-ion battery technologies.
Among a number of potential replacements of the state-of-the-art lithium salt (LiPF6), lithium difluoro(oxalate)borate (LiODFB or LiC2O4BF2) has exerted a tremendous fascination on researchers because LiODFB, a hybrid salt of lithium bis(oxalate)borate(LiBOB) and lithium tetrafluoroborate(LiBF4), possesses beneficial properties of both parent anions [7, 9, 13–16]. These merits include 1) a relatively high ionic conductivity over a wide temperature range, 2) the ability to not only support metallic lithium cycling reversibly on the surface of a copper anode current collector, but also passivate the aluminum cathode current collector at high voltage, 3) the active formation of a SEI layer, and 4) the possibility to increase battery safety protection and overcharge tolerance. However, the commercialization of LiODFB is limited by the high cost of preparation or the difficulty in the separation and purification during the synthetic process when pervious routes were adopted.
LiODFB was first synthesized by LiBF4 and lithium oxalate (H2C2O4) in the medium of an aprotic solvent, such as dimethyl carbonate (DMC) with a poor mass conversion rate below 85% [16]. The relatively pure LiODFB could be synthesized by the chemical reaction between H2C2O4 and LiBF4 in an acetonitrile (AN) solvent with the catalyzer of AlCl3 or SiCl4, by which the LiBF4 impurity was decreased to 0.5% [17]. While the by-product, hydrogen chloride (HCl) was also brought in. Therefore, this synthetic way made a stringent request of the reaction equipment. To overcome these shortcomings, LiODFB (>99.9%) could be synthesized by the direct reaction of boron trifluoride diethyl etherate (BF3-ether) with H2C2O4 (1:1, in molar) in DMC solvent [7, 13–15]. But half the lithium sources of Li2C2O4 were wasted on the undesirable byproduct as LiF. To make matters worse, the resulting LiF would coat upon the surface of Li2C2O4 particles, which would cut off the reaction between Li2C2O4 and BF3-ether. Furthermore, the resulting LiF can react with BF3-ether to form LiBF4, which would reduce the reaction selectivity [18]. In addition, our previous study suggested that setting the molar ratio of BF3-ether and H2C2O4 as 2:1 can decrease the by-effect of LiF and improve the whole process controllability with a co-product of LiBF4 [19, 20].
It can be concluded that all of these methods still face with a common challenge, the separation with a certain amount of LiBF4 that derives from reagent or product. Unfortunately, the knowledge of phase diagrams about LiX(X=ODFB–, BF4–)-aprotic solvent binary systems is insufficient currently [21–24], not to mention the LiODFB-LiBF4-solvent ternary systems, which is important to guide the design of recrystallization separation process. Meanwhile, the study about ions coordination (ions solvation and association) in molecular level that usually largely governs the macro-level thermal phase behavior falls short as well [25–27]. As a result, the commonly adopted repeating crystallization approach is very time-consuming as well as material-wasting. More importantly, both phase diagram and ion coordination are important for finding the operational liquid range and detecting any anomaly at low temperatures (glass transition, crystalline solvate) or for predicting the properties of concentrated solutions, including ionic conductivity, density, viscosity, etc [28, 29]. In brief, the link between molecular-level ion/solvent structure and macro-level thermal phase behavior can determine, in part, the performance of Li-ion batteries. As a readily accessible solvent for LiODFB purification and LiODFB-based electrolyte formulation, DMC is of principal interest in the present body of research among a series of LiODFB-solvent systems.
The present study, therefore, has focused on exploring a simple and cheap method for synthesis of LiODFB to reduce or avoid the existence of LiBF4, and determining the thermal phase diagram and the ions coordination of LiODFB-DMC binary system to optimize the recrystallization of LiODFB and provide fundamental information for LiODFB- based electrolyte designing.
2 Experimental
2.1 Synthesis and purification of LiODFB
BF3-dimethyl carbonate (BF3·DMC, 99%), H2C2O4 (99%) and Li2CO3 (99%) were used as-received from Sigma-Aldrich, and dimethyl carbonate (DMC, 99%) was on standby after a rigorous distillation process. LiODFB (≥99.95%) was synthesized by the two-step as:
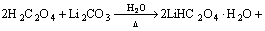
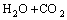 (1)
(1)
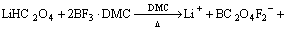
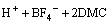 (2)
(2)
In the first synthesis step, LiHC2O4·H2O was synthesized by adding Li2CO3 powder into H2C2O4 solution slowly, then stirring the mixture at 55–65 °C for 3 h and cooling crystallization above the freezing point of H2O for 24 h. In particular, it is deserved to be mentioned that the molar ratio of H2C2O4 to Li2CO3 was typically slightly greater than 2:1 to avoid the formation of Li2C2O4. Next, pure LiHC2O4 was obtained by suction filtration and vacuum drying at 120 °C. Lastly, LiHC2O4 powder was sealed in a sealed container after milling in a N2-filled vacuum atomspheres inert atmosphere glovebox (<1×10–6 H2O). Besides, the crystalline phase of the synthesized material was identified by powder X-ray diffraction measurement (Rigaku, D/max-2500, Japan) using Cu Kα radiation (30 kV, 20 mA).
In the second step, LiHC2O4, BF3·DMC and DMC were mixed with a mole ratio of 1:2:6. After heating and stirring at a hermetically-sealed polyethylene flask at 80 °C for 2–3 h, supersaturated LiODFB-DMC mixture containing liquidus HBF4 (Tm at –90 °C, Tb at 130 °C) can be gained. Obviously, the separation of LiODFB(s) and HBF4(l) is much easier than that of LiODFB(s) and LiBF4(s). Next, mixtures were cooled slowly and kept still at least 24 h after seed was added (viz. the 1st recrystallization). Most impurities such as unreacted BF3, HBF4 and the hydrolysis byproducts were filtered off. In subsequent steps, the crude lithium salt, a mixture that contains a little bit of impurities introduced by the hydrolysis with trace amount of water in row materials as well as inevitable ion solvation and association among H+(or Li+), BF4– and DMC molecules, were then recrystallized a total three times in DMC by supersaturing the solution, vacuum filtering, and allowing the solution to slowly cool to promote large crystal growth under the guidance of LiODFB-DMC phase diagram presented in this work. After recrystallization, LiODFB solvate (LiODFB·(DMC)3/2, Tm at 84 °C [30]) was dried at 105 °C for 48 h to remove the DMC, yielding a high purity salt. All actions were performed in a N2 glovebox. The water content was determined by a JB-1A interference-free coulometric titration measurement with Karl Fisher reagents based on N-methylformamide (Aldrich, US).
2.2 Characterization of LiODFB
In order to verify the purity of the LiODFB, firstly, the salt was dissolved in Acetonitrile-D3 (CD3CN) for NMR analysis. 11B and 11F NMR were performed on a Bruker AVANCE 500 MHz spectrometer; secondly, impurities of metal ions were determined by Inductively Coupled Plasma- Atomic Emission Spectrometry (ICP-AES) measurement (Perkin Elmer Optima5300, USA). To confirm the crystalline structure of LiODFB, Fourier transform infrared spectroscopy (FTIR) measurement and Raman spectroscopy were carried out using a Nicolet 6700 FTIR spectrometer and LabRam Aramis Raman spectroscopy (Horiba Jobin-Yvon, France), respectively.
2.3 Sample preparation for DSC and Raman study
Mixtures were prepared in the glovebox by combining appropriate amounts of the LiODFB salt with DMC in hermetically-sealed glass vials and then heated/stirred on a Thermo Shaker (TCS10, Ruicheng Instr. Co., Ltd., China) to form a homogenous solution. The composition of each mixture/solvate is described using the following three notations: (1–x)DMC–(x)LiODFB, (DMC)n– LiODFB and LiODFB·(DMC)n for discussions focusing on mole fraction, ratio of DMC/Li and specific crystalline solvates, respectively.
2.4 Thermal measurement
Differential scanning calorimeter (Perkin Elmer, Pyris Diamond, USA) with liquid N2 cooling was used for the DSC measurements. The instruments were calibrated with n-octane (Tm at –56.80 °C) and indium (Tm at 156.60 °C). Two or three droplets of sample (≈5 mg) were added to an aluminum pan and hermetically-sealed in the glovebox. Typically, it was necessary to cycle and repeatedly anneal the sample pans at subambient temperature to ensure complete crystallization of the samples. Once the samples were crystallized, the pans were cooled to –100 °C and then heated (5 °C/min) to the melting and/or the decomposition temperature of sample. Peak temperature values were recorded from the final heating thermograms and these data were used to construct the reported phase diagrams.
TGA measurements were performed on a TA Instruments SDTQ600 thermogravimetric analyzer by heating from 20 to 500 °C at a rate of 5 °C/min. The TGA furnace was purged with N2 gas during the measurements. DSC and TGA measurements were performed in duplicate to ensure reproducibility.
2.5 Raman spectroscopy
A Horiba Jobin-Yvon LabRam Aramis Raman spectroscopy was used to collect Raman vibrational spectra using a 532 nm–1 semiconductor laser as the exciting source and a hermetically-sealed Linkam heating/cooling stage for temperature control. Samples were sealed in disposable optical glass test-tubes in the glovebox. Spectra were typically collected with a 50× long-range objective using a 20 s exposure time and 20× accumulation to ensure that high resolution spectra were obtained. Raman spectra were evaluated with LabSpec software. Average solvation number (N), the fraction of solvent molecules coordinated to Li+ cations can be determined from the following relation [21, 24]:
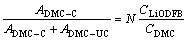 (3)
(3)
where ADMC–C and ADMC–UC are the integrated area intensities of the bands for the coordinated and uncoordinated DMC, respectively, CLiODFB and CDMC are the concentrations of the salt and DMC, respectively, and N is the average solvation number.
3 Results and discussion
3.1 Analysis of synthesis process
Figure 1 shows the XRD patterns of LiHC2O4 powders prepared from 1st synthesis step. It is observed that all fundamental peaks can be indexed to the lithium hydrogen oxalate hydrate (LiHC2O4·H2O, JCPDS#49-1209). The crystal water is resulted from the absorption of moisture during sample preparation and sample testing. Hence, the XRD result proves the feasibility of Eq.(1).
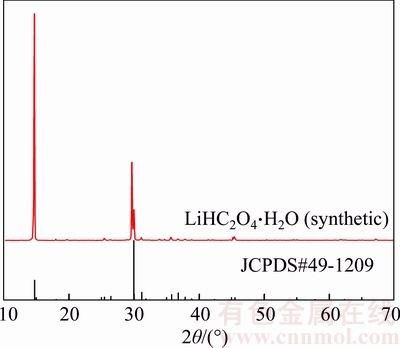
Figure 1 X-ray diffraction (XRD) pattern of LiHC2O4 synthesized by 1st step
Figure 2 displays the 11B and 19F NMR results for LiODFB at different stages, respectively. The 11B NMR spectra of the first recrystallized LiODFB after decanting and rinsing reveal that the course of Eq. (2) leads to the formation of LiODFB-rich mixtures of salt with a purity up to 95 % (mass fraction). Moreover, after 4 times recrystallization, the content of impurities including HBF4 [31] (and/or LiBF4 [32–34]) and other hydrolysis impurities (located at about 20×10–6, 7.5×10–6 and 4.2×10–6) were decreased speedily, and the next 11B and 19F NMR spectra (Figures 2(h) and (i)) yield no detectable boracic and/or fluoro impurities of the end-product LiODFB. Furthermore, the relative content of LiODFB in raffinate rises with the recrystallization times.
Hence, this new synthesis method which can cut 2–3 recrystallization times than others in previous reports, will improve the yield of high-quality LiODFB effectively. Besides, the purity of LiODFB as-synthesized also has been verified by the chemical analysis of ion contents: each of their impurity contents were below 2.1×10–6 for water, and 1.4×10–6, 15.3×10–6, 9.8×10–6, 8.1×10–6 and 12.5×10–6 for metal ions Mg, Na, Zn, Fe and Ca, respectively. Therefore, it can be observed that the two-step reaction suggested by this study can significantly simplify the recrystallization process, lower the preparation cost and obtain high quality LiODFB efficiently as well.
3.2 Characterization of LiODFB as-synthesized
The experimental FTIR and Raman spectrum for anhydrous LiODFB crystalline salt are shown in Figure 3. To aid in the assignment of the peaks, the DFT computed vibrational modes for the uncoordinated ODFB– anion reported by HAN et al [30] are also listed in Table 1.
The two strong peaks found above 1700 cm–1 in Figure 3(a) correspond to the C=O stretching vibrations-antisymmetric and symmetric, respectively. According to the AGG-IIIb structure, LiODFB has carbonyl oxygen atoms coordinated to three Li+ cations, one of which is coordinated by both carbonyl groups. This coordination within the LiODFB crystal structure may result in the single Raman peak at 1405 cm–1, corresponds to the combination band of the C—C and symmetric C—O stretching modes. While the peak observed at about 946 cm–1 is attributed to combination of symmetric B—O, C—C and B—F stretching modes. The very strong sole peak near 722 cm–1 corresponds to a ring breathing mode, which is predicted to be the third strongest peak in the Raman spectra by the calculations. The two or more peaks observed at 600–640 cm–1 are most likely due to a combination of different O—C—C bending modes and B—F scissoring from the other half of the ODFB– anion. From the above analysis, the structure of the end-product also agrees well with this of LiODFB in previous literatures [15, 30]. In summary, it should be surely believed that the end product of the described synthetic method is lithium difluoro(oxalate)borate (LiODFB or LiBC2O4F2).
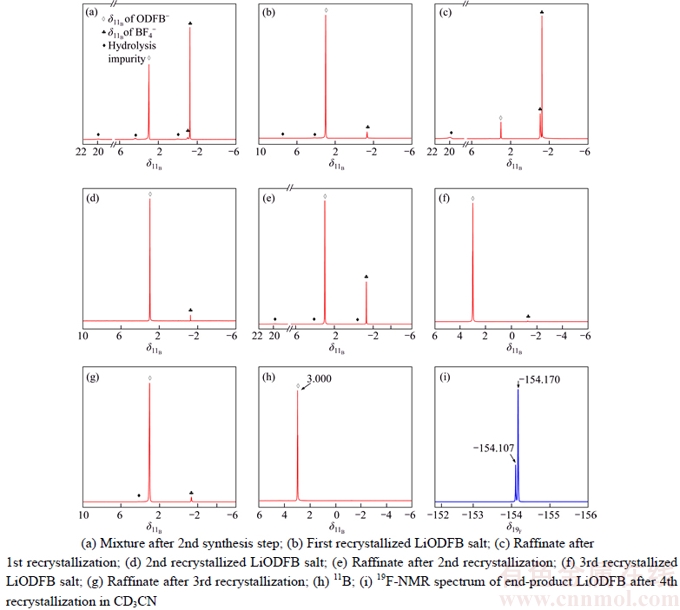
Figure 2 11B-NMR spectra:
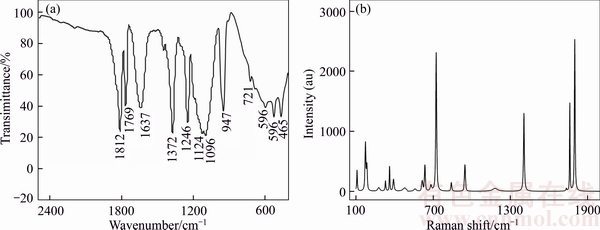
Figure 3 FTIR spectrum (a) and Raman spectrum (b) of LiODFB as-prepared (at 20 °C)
Table 1 Calculated vibration mode frequencies (Fc), Raman activities (Ar) and IR intensities (Iir), and tentative assignments [29] compared to experimental data
The thermal property of LiODFB is tested by the DSC and TGA measurements (Figure 4). It appears from DSC-TGA analysis that LiODFB·H2O [30] and LiODFB·(H2O)2 [15] might be formed when LiODFB was exposed to ambient moisture during the Al2O3 sample pan transferring to the test board, and the endothermic peaks at 128 °C and 159 °C would reflect the decomposition of crystal water. Furthermore, LiODFB sample displayed an endothermic peaks at 269 °C and great mass loss during this period, which can be attributed to the multistep decomposition of LiODFB. According to pervious literatures, LiODFB maybe present a combination of two decomposition mechanisms for both LiBF4 and LiBOB (Eqs. (4), (5) [35], (6) [36]):
 →
→ (4)
(4)
 →
→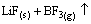 (5)
(5)
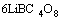 →
→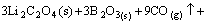
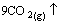 (6)
(6)
Li2C2O4+3B2O3→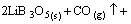 CO2(g)↑(7)
CO2(g)↑(7)
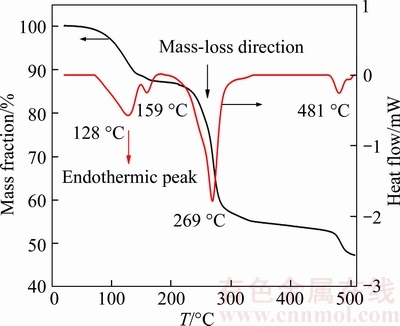
Figure 4 DSC and TGA heating traces (5°C/min) of LiODFB as-prepared
The final LiODFB decomposition temperature step may involve the decomposition of the remaining lithium oxalate (Eq. (7)) due to its peak position of about 481 °C near the reported LiBOB decomposition temperature of about 520 °C as well [36]. To sum up, LiODFB exerts both superior water tolerance and thermal stability compared to traditional lithium salt (e.g. LiPF6), which can greatly improve battery performance and safety.
3.3 Phase behavior of LiODFB-DMC binary system
Phase diagram is not only of great benefit to optimize the recrystallization separation process of LiODFB, but also of great value for the researches to investigate the special phenomenon (e.g., glass transition and crystalline solvate) of electrolyte at low temperature and guide the design of electrolyte formulations. Representative DSC heating traces examples and the corresponding phase diagrams of (DMC)n-LiODFB mixtures are given in Figures 5(a), (b) and (c), respectively. According to the DSC heating traces of all tested samples, the phase diagram was prepared by plotting the peak temperature (Tp) for thermal events such as solid–solid phase transition and the melt transition when the thermal peak is very sharp and picking the extrapolated final temperature (Tef) as the liquidus point. Only a small portion of the (DMC)n–LiODFB phase diagram was examined due to the limited solubility of this salt in DMC.
From Figures 5(a) and (c), pure DMC has a Tm at 4.6 °C and a low energy solid–solid phase transition at –53 °C [37]. With the addition of LiODFB, the solvate peaks get smaller and shift to lower temperatures due to the availability of less bulk solvent for crystallization. No solvate crystalline phases are observed for the (DMC)n– LiODFB mixtures when n≥12. Hence, it also can be explained why the raffinate always contains a certain amount of LiODFB no matter how recrystallization times perform. However, as the concentration of LiODFB increases continually, new phases start to form, gives only one distinct phases for 1.5/1 composition with a melting transition at 84 °C. The crystalline solvate structure of LiODFB·(DMC)3/2 was previously reported by HAN et al [30]. It is noteworthy that LiODFB crystallizes as LiODFB·(DMC)3/2 even in an dilute solution which will be discussed in the next discussion.
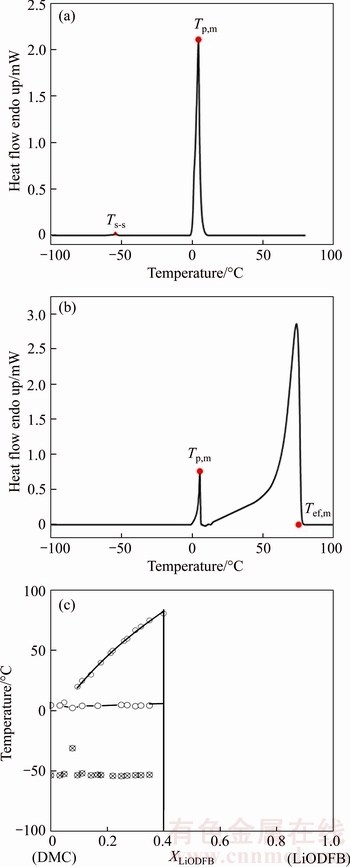
Figure 5 DSC heating traces (5 °C/min) of pure DMC (a) and DMC-LiODFB=3:1 mixtures (b) and corresponding phase diagrams for LiODFB–DMC binary system (c)
3.4 Raman characterization of (DMC)n–LiODFB binary system
Raman spectroscopic analysis can give an insight into ion solvation and ionic association in the salt-solvent system, which does often largely govern the phase behavior of LiODFB-DMC binary system [25–27]. In DMC solvent with a small dielectric constant (ε=3.11 [37]), ion association is the dominant form of molecular interaction and DMC molecules is ordered and coordinated to Li+ cations by the carbonyl oxygen (C=O) atom or one of the other oxygen (C—O—C) atoms, resulting in the shift of DMC CH3—O stretch bands (about 918 cm–1) [38]. Upon adding LiODFB to DMC, the Raman spectra of the DMC CH3—O stretch bands were spilt into two components at about 926 and 935 cm–1 that belonged to CIPs and aggregate (AGG-IIa), respectively, and the line at about 942 cm–1 corresponded to the unionized LiODFB as AGG-IIIb in solution (Figure 6). The AGGs coordination, including AGG-IIa and AGG- IIIb has been investigated in crystalline solvate by ALLEN et al [15] and HAN et al [30]. However, detail information about CIPs needs thorough investigation in our next study.
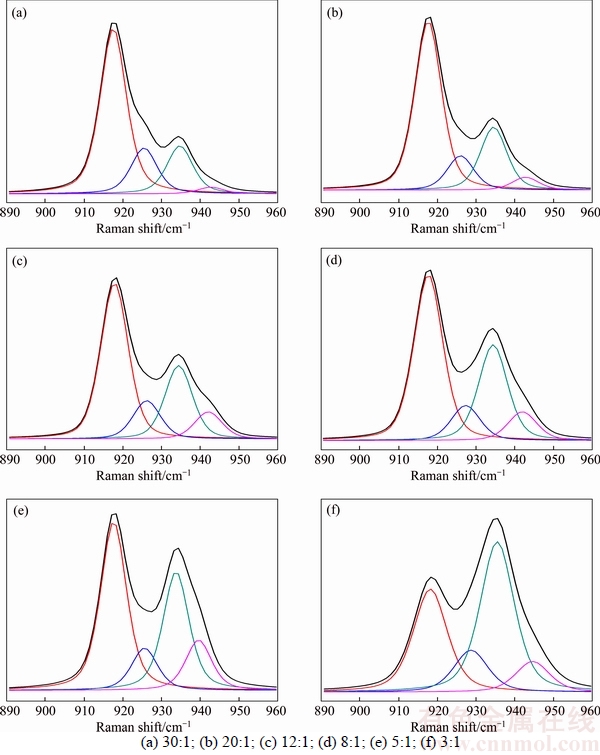
Figure 6 Raman spectra at 20 °C of DMC CH3—O stretching mode (about 918 cm–1) for (DMC)n–LiODFB mixtures with different DMC/LiODFB ratios:
Based upon the data shown in Figure 6, the ion pair content variations were prepared according to the change of peaks intensities (Figure 7(a)). As presented in Figure 7(a), the concentration dependence of the species assigned to the cations in AGG-IIIb passed through a maximum (DMC:LiODFB=5:1), while the cations in AGG-IIa (LiODFB·(DMC)3/2) continued to rise with the concentration of LiODFB and became the dominant type in the concentrated solution. This phenomenon can also explain the poor conductivity of DMC-LiODFB mixtures. Combining the information above and the molar ratio of DMC to LiODFB, the N value variation with salt concentration was determined, which gave an indication of the fraction of solvent molecules coordinated to Li+ cations (Figure 7). For the most dilute solutions, analytical errors will be the greatest, so N values for data with x<0.10 or thereabout are considered to be unreliable. Hence, in DMC- LiODFB binary system, the Li+ cation has a mean coordination by two DMC molecules, which is in accordance with the findings by HAN et al [30].
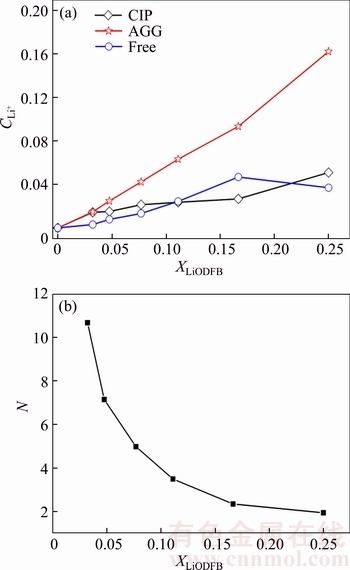
Figure 7 Anion and ion pair content in (DMC)n– LiODFB mixtures (a) and mean number of solvent molecules bonded to cations in (DMC)n–LiODFB mixtures (b)
From above discussions in molecular interaction level, it is drawn that there are at least three thermodynamically stable phases that may be formed, CIPs, AGG-IIa and AGG-IIIb, but their formation is strongly dependent upon nucleation kinetics. It seems that the solvent and the anions fail to pack in a favorable way to form an ordered crystalline structure as CIPs or AGG-IIIb from solution. Thus, it is proposed that (DMC)n–LiODFB mixtures tends to form a LiODFB·(DMC)3/2 phase, even in dilute mixtures.
4 Conclusions
Lithium difluoro(oxalato)borate (LiODFB) is a promising salt for lithium ion batteries owing to its unique properties. To accelerate its large-scale application, the exploring of a simple synthesis method and a comprehensive understanding about LiODFB-DMC binary system are of principal interest.
In this work, LiODFB (>99.95%) are synthesized by an improved two-step method, which is cheaper, simpler and more environmentally friendly than any previous method. During the process, LiODFB is easily isolated with HBF4(l) compared with LiBF4(s), whose solubility is similar to LiODFB. The vibrational spectroscopy information of as-prepared LiODFB has been determined by FTIR and Raman, which is in accordance with other literatures. Based upon the DSC and TGA analysis of LiODFB salt, the decomposition temperature of LiODFB is up to about 270 °C and its decomposition mechanism may be a combination of LiBF4 and LiBOB. Besides, the endothermic peaks located at about 130 °C and about 160 °C should attribute to the loss of crystal water, which reflects that LiODFB has a lower water sensitivity than LiPF6.
Moreover, the phase diagram and Raman spectra of LiODFB-DMC binary system have been obtained to optimize the synthesis and purification process of LiODFB, which gives an insight to macro phase behavior and molecular interaction between solute and solvent. From these information, there are three types of molecular interaction form in LiODFB-DMC binary system (CIPs, AGG-IIa, AGG-IIIb), however, LiODFB can only be isolated as large single crystal solvate as LiODFB·(DMC)3/2 by slowly cooling subjected to the nucleation kinetics, which indicates that the solvate formation from LiODFB-DMC system may be a function of more than the ionic association tendency of the anions. In addition, LiODFB crystal solvate can hardly isolate from relative dilute solution (the value of DMC/Li+ is greater than 12), which means that roto-evaporation of mixtures, mother liquid recovery and recrystallization times cut by process control to grow grand single crystal are very useful approaches to improve the yield of LiODFB. Hence, it can be concluded that our work is of great help to guide the LiODFB synthesis and recrystallization process.
References
[1] REDDY T B. Linden’s handbook of batteries, fourth edition [M]. New York: McGraw-Hill, 2011.
[2] SCROSATI B. Challenge of portable power [J]. Nature, 1995, 373(6515): 557–558.
[3] WHITTINGHAM M S. Lithium batteries and cathode materials [J]. Chemical Reviews, 2004, 104(10): 4271–4302.
[4] GOODENOUGH J B, KIM Y. Challenges for rechargeable Li batteries [J]. Chemistry of Materials, 2010, 22(3): 587–603.
[5] HU Meng, PANG Xiao-li, ZHOU Zhen. Recent progress in high-voltage lithium ion batteries [J]. Journal of Power Sources, 2013, 237: 229–242.
[6] SMART M C, KRAUSE F C, WEST W C, SOLER J, PRAKASH G K S, RATNAKUMER B V. Development of Li-ion battery electrolytes with improved safety for NASA applications [EB/OL]. [2016–05–20]. http://ma.ecsdl.org/ content/MA2010-01/3/167.full.pdf.
[7] ZHANG Sheng-shui. An unique lithium salt for the improved electrolyte of Li-ion battery [J]. Electrochemistry Communications, 2006, 8(9): 1423–1428.
[8] LI Chun-lei, ZHAO Yang-yu, ZHANG Hong-ming, LIU Jin-liang, JING Jie, CUI Xiao-ling, LI Shi-you. Compatibility between LiNi0.5Mn1.5O4 and electrolyte based upon lithium bis(oxalate)borate and sulfolane for high voltage lithium-ion batteries [J]. Electrochimica Acta, 2013, 104:134–139.
[9] ZHOU Hong-ming, XIAO Kai-wen, LI Jian. Lithium difluoro-(oxalate)borate and LiBF4 blend salts electrolyte for LiNi0.5Mn1.5O4 cathode material [J]. Journal of Power Sources, 2016, 302: 274–282.
[10] SIVAKUMAR P, NAYAK P K, MARKOYSKY B, AURBACH D, GEDANKEN A. Sonochemical synthesis of LiNi0.5Mn1.5O4 and its electrochemical performance as a cathode material for 5 V Li-ion batteries[J]. Ultrasonics Sonochemistry, 2015, 26: 332–339.
[11] SHIEH D, HSIEH P, YANG M. Effect of mixed LiBOB and LiPF6 salts on electrochemical and thermal properties in LiMn2O4 batteries [J]. Journal of Power Sources, 2007, 174(2): 663–667.
[12] XIANG H F, WANG H, CHEN C H, GE X W, GUO S, SUN J H, HU W Q. Thermal stability of LiPF6-based electrolyte and effect of contact with various delithiated cathodes of Li-ion batteries [J]. Journal of Power Sources, 2009, 191(2): 575–581.
[13] ZHANG Sheng-shui. Lithium oxalyldifluoroborate as a salt for the improved electrolytes of Li-Ion batteries [J]. The Electrochemical Society Transactions, 2007, 3(27): 59–68.
[14] LIU Jun, CHEN Zong-hai, BUSKING S, AMINE K. Lithium difluoro(oxalato)borate as a functional additive for lithium-ion batteries[J]. Electrochemistry Communications, 2007, 9(3): 475–479.
[15] ALLEN J L, HAN S D, BOYLE P D, HENDERSON W A. Crystal structure and physical properties of lithium difluoro(oxalato)borate (LiDFOB or LiBF2Ox) [J]. Journal of Power Sources, 2011, 196(22): 9737–9742.
[16] TSUJIOKA S, TAKASE H, TAKAHASHI M, SUGIMOTO H, KOIDE M. electrolyte for electrochemical device: US, 6787267B2 [P]. 2004.
[17] TSUJIOKA S, TAKASE H, TAKAHASHI M. Process for synthesizing ionic metal complex: US, 6849752B2 [P]. 2005.
[18] LI Shi-you, ZHAO Wei, CUI Xiao-ling, ZHAO Yang-yu, LI Bu-cheng, ZHANG Hong-ming, LI Yong-li, LI Gui-xian, YE Xiu-shen, LUO Yong-chun. An improved method for synthesis of lithium difluoro(oxalato)borate and effects of sulfolane on the electrochemical performances of lithium-ion batteries [J]. Electrochimica Acta, 2013, 91: 282–292.
[19] ZHOU Hong-ming, LIU Fu-rong, LI Jian LI Yan-fen, ZHU Yu-hua, FANG Zhen-qi. Hydrolysis and influences on physical and chemical properties for lithium battery electrolyte LiODFB [J]. Journal of Central South University: Science and Technology, 2012, 43(11): 4228–4233. (in Chinese)
[20] ZHOU Hong-ming, FANG Zhen-qi, LI Jian. LiPF6 and lithium difluoro (oxalato)borate/ethylene carbonate+ dimethyl carbonate+ethyl(methyl)carbonate electrolyte for Li4Ti5O12 anode [J]. Journal of Power Sources, 2013, 230: 148–154.
[21] SEO D M, BORODIN O, HAN S D, LY Q, BOYEL P D, HENDERSON W A. Electrolyte solvation and ionic
[22] SEO D M, BOYLE P D, HENDERSON W A. Poly [[(acetonitrile)lithium(I)]-μ3-tetrafluoridoborato] [J]. Acta Crystallo-graphica Section E–Structure Reports Online, 2011. DOI: 10.1107/S1600536811012141.
[23] GAFIROV M M, KIRILLOV S A, GOROBETS M I, RABADANOV K S, ATAEV M B, TRETYAKOV D O, AYDEMIROV K M. Phase equilibria and ionic solvation in the lithium tetrafluoroborate-dimethylsulfoxide system [J]. Journal of Applied Spectroscopy, 2015, 81(6): 912–918.
[24] HAN S D, BORAODIN O, ALLEN J L, SEO D M, MCOWEN D W, HENDERSON W A. Electrolyte solvation and ionic association. IV. Acetonitrile-lithium difluoro(oxalato)borate (LiDFOB) mixtures [J]. Journal of The Electrochemical Society, 2013, 160(11): A2100–A2110.
[25] KIRILLOV S A, GAFUROV M M, GOROBETS M I, ATAEV M B. Raman study of ion pairing in solutions of lithium salts in dimethyl sulfoxide, propylene carbonate and dimethyl carbonate [J]. Journal of Molecular Liquids, 2014, 199: 167–174.
[26] GOROBETS M I, ATAEV M B, GAFUROV M M, KIRILLOV S A. Raman study of solvation in solutions of lithium salts in dimethyl sulfoxide, propylene carbonate and dimethyl carbonate [J]. Journal of Molecular Liquids, 2015, 205: 98–109.
[27] KIRILLOV S A, GOROBETS M I, TRETYAKOV D O, ATAEV M B, GAFUROV M M. Phase diagrams and conductivity of lithium salt systems in dimethyl sulfoxide, propylene carbonate and dimethyl carbonate [J]. Journal of Molecular Liquids, 2015, 205: 78–84.
[28] PERRON G, COUTURE L, LAMBERT D, DESNOYERS J E. Phase diagrams, molar volumes, heat capacities, conductivities and viscosities of some lithium salts in aprotic solvents [J]. Journal of Electroanalytical Chemistry, 1993, 355: 277–296.
[29] SEO D M, BORODIN O, BALOGH D, O’CONNELL M, LY Q, HAN S D, PASSERINI S, HENDERSON W A. Electrolyte solvation and ionic association III. Acetonitrile- Lithium salt mixtures–transport properties [J]. Journal of the Electrochemical Society, 2013, 160(8): A1061–A1070.
[30] HAN S, ALLEN J L, J NSSON E, JOHANSSON P, MCOWEN D W, BOYLE P D, HENDERSON W A. Solvate structures and computational/spectroscopic characterization of lithium difluoro(oxalato)borate (LiDFOB) electrolytes [J]. The Journal of Physical Chemistry C, 2013, 117(11): 5521–5531.
NSSON E, JOHANSSON P, MCOWEN D W, BOYLE P D, HENDERSON W A. Solvate structures and computational/spectroscopic characterization of lithium difluoro(oxalato)borate (LiDFOB) electrolytes [J]. The Journal of Physical Chemistry C, 2013, 117(11): 5521–5531.
[31] STUNGIS G E. NMR of HBF4 [J]. The Journal of Chemical Physics, 1971, 55(1): 263.
[32] PLAKHOTNIK V N, ERNST L, SAKHAII P, TOVMASH N F, SCHMUTZLER R. Interparticle interaction in lithium tetrafluoroborate solutions [J]. Journal of Fluorine Chemistry, 1999, 98(2): 133–135.
[33] VOIGT N, W LLEN V L. The effect of plastic-crystalline succinonitrile on the electrolyte system PEO:LiBF4: Insights from solid state NMR [J]. Solid State Ionics, 2014, 260: 65–75.
LLEN V L. The effect of plastic-crystalline succinonitrile on the electrolyte system PEO:LiBF4: Insights from solid state NMR [J]. Solid State Ionics, 2014, 260: 65–75.
[34] VOIGT N, W LLEN V L. The mechanism of ionic transport in PAN-based solid polymer electrolytes [J]. Solid State Ionics, 2012, 208: 8–16.
LLEN V L. The mechanism of ionic transport in PAN-based solid polymer electrolytes [J]. Solid State Ionics, 2012, 208: 8–16.
[35] SHANTHI M, MATHEW C M, ULAGANATHAN M, RAJENDRAN S. FT-IR and DSC studies of poly(vinylidene chloride-co-acrylonitrile) complexed with LiBF4 [J]. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 2013, 109: 105–109.
[36] ZINIGRAD E, LARUSH-ASRAF L, SALITRA G, SPRECHER M, AURBACH D. On the thermal behavior of Li bis(oxalato)borate LiBOB [J]. Thermochimica Acta, 2007, 457(1, 2): 64–69.
[37] XU K. Nonaqueous liquid electrolytes for lithium-based rechargeable batteries [J]. Chemical Reviews, 2004, 104(10): 4303–4418.
[38] AFROZ T, SEO D M, HAN S D, BOYLE P D, HENDERSON W A. Structural interactions within lithium salt solvates: Acyclic carbonates and esters [J]. The Journal of Physical Chemistry C, 2015, 119(13): 7022–7027.
(Edited by FANG Jing-hua)
中文导读
二氟草酸硼酸锂(LiODFB)的制备及其在碳酸二甲酯中的相图和离子缔合规律
摘要:本文介绍了一种新型两步合成法合成二氟草酸硼酸锂,简化了LiODFB的重结晶分离过程。通过NMR、ICP-AES以及卡尔费休法测定所制备的LiODFB的纯度,结果表明,制备的LiODFB纯度高达99.95%,所含金属离子及水分均得到了很好的控制。此外,采用FTIR、Raman和DSC-TGA分析了LiODFB的结构信息及热力学性质,发现LiODFB具有非常优异的热力学稳定性和较低的水分敏感性, 并且其结构信息与文献报道一致。另外,结合相图以及拉曼光谱技术研究了LiODFB-DMC二元体系的热力学相转变和离子规律,用来指导优化LiODFB的合成及重结晶工艺。结果表明,尽管在LiODFB-DMC二元体系中存在3种类型的分子间相互作用形式(CIPs,AGG-IIa,AGG-IIIb),但受成核动力学条件影响,LiODFB仅可以通过缓慢冷却的方式获得较大的单晶溶剂化物—LiODFB·(DMC)3/2。本文所提供的基础数据有助于推动LiODFB在锂离子电池领域的应用。
关键词:二氟草酸硼酸锂;两步合成法;重结晶;相图;离子缔合
Foundation item: Project(51371198) supported by the National Natural Science Foundation of China; Project(K1202039-11) supported by the Science and Technology Project of Changsha, China
Received date: 2016-06-19; Accepted date: 2016-09-28
Corresponding author: LI Jian, PhD, Associated Professor; Tel: +86–731–88877173; E-mail: ziliao2000@126.com; ORCID: 0000-0002- 3446-6963
Abstract: A new two-step synthetic method was successfully developed to simplify the recrystallization process of lithium difluoro(oxalate)borate (LiODFB). Meanwhile, the purity of LiODFB as-prepared was determined by NMR, ICP-AES and Karl Fisher measurements, respectively. The as-prepared LiODFB presents a high purity up to 99.95%. Its metal ions and water contents are under good control as well. Besides, its structure information and thermal properties were confirmed by FTIR, Raman and DSC-TGA analyses, respectively. LiODFB exerts fine thermostability and hypo-water-sensitivity and its structure information agrees well with previous literature. Furthermore, a combination of phase diagram and Raman spectroscopy were utilized to study the thermal phase behavior and ions coordination of LiODFB-DMC binary system to optimize the synthesis and recrystallization process. Although there are three types of molecular interaction forms (CIPs, AGG-IIa, AGG-IIIb) in LiODFB-DMC binary system, LiODFB can only be isolated as large single crystal solvate as LiODFB·(DMC)3/2 by slowly cooling subjected to the nucleation kinetics. Therefore, the fundamental information of our work is helpful in accelerating the application of LiODFB in Li-ion secondary batteries.


