- Abstract:
- 1 Introduction▲
- 2 Experimental section▲
- 3 Results and discussion▲
- 4 Conclusions▲
- References
- Figure
- Fig.1 Shear stress vs shear rate (a), apparent viscosity vs shear rate (b) for 20 kGy irradiated [Me3NC2H4OH]+[Zn2Cl5]- at different temperatures
- Fig.2 Shear stress vs shear rate (a), apparent viscosity vs shear rate (b) for 20 kGy irradiated [Me3NC2H4OH]+[Zn3Cl7]- at different temperatures
- Fig.3 Plots of viscosity as functions of temperature and irradiation doses: (a) [Me3NC2H4OH]+[Zn2Cl5]-, 298.15-333.15 K; (b) [Me3NC2H4OH]+[Zn3Cl7]-, 318.15-343.15 K
J. Cent. South Univ. Technol. (2008) 15(s1): 145-148
DOI: 10.1007/s11771-008-334-y
![]()
Rheological properties of gamma irradiated [Me3NC2H4OH]+[Zn2Cl5]- and [Me3NC2H4OH]+[Zn3Cl7]- ionic liquids
LI Qian-mei(李倩妹)1,2, LUO Ying-she(罗迎社)1, WU Guo-zhong(吴国忠)3, CHEN Shi-mou(陈仕谋)3
(1. Institute of Rheological Mechanics and Material Engineering, Central South University of Forestry and
Technology, Changsha 410004, China;
2. Institute of Civil Architecture and Mechanics, Central South University of Forestry and Technology,
Changsha 410004, China;
3. Shanghai Institute of Applied Physics, Chinese Academy of Sciences, Shanghai 201800, China)
Abstract:
The objective of this work is to verify the rheological behavior of irradiated [Me3NC2H4OH]+[Zn2Cl5]- and [Me3NC2H4OH]+[Zn3Cl7]- ionic liquids in comparison to the unirradiated ones, the viscosities were measured by a strain-control experiment under different irradiation doses’ samples(0, 10, 20, 50, 100 kGy) at different shear rates and temperatures. The curves of shear stress against shear rate present that the viscosity of ionic liquid is insensitive to shear rate; the viscosity of ionic liquids decreases with increasing temperature, and can be fitted by Arrhenius equation very well. Gamma radiation causes a decrease of viscosity of [Me3NC2H4OH]+[Zn3Cl7]- by greater than 10%, but it does not impair the viscosity of [Me3NC2H4OH]+[Zn2Cl5]- (within the experimental error) except 20 kGy irradiated sample. The results show that the radiation stability of [Me3NC2H4OH]+[Zn2Cl5]- is higher than that of [Me3NC2H4OH]+[Zn3Cl7]-.
Key words:
ionic liquid; rheological property; viscosity; gamma radiation; Arrhenius equation;
1 Introduction
Room temperature ionic liquids (RTILs) are composed of cation and anion and they are liquids at room temperature. Due to their unique properties including non-volatility, high polarity, negligible vapor pressure, large electrochemical window, ease of recycling and increased selectivity and chirality, ionic liquids received increased attention[1-3]. As a kind of novel solvent, RTILs are widely used in a variety of chemical and engineering processes.
In radiation chemistry, RTILs have been applied as the solvents of radiation polymerization of different monomers. YANG and DIONYSIOU[4] studied the photolytic degradation of chlorophenol and its deriva- tives in [Bmim+][PF6-] and [emim+][beti-] ionic liquids upon UV irradiation. GELESKY et al[5] prepared stable Pd and Rh nanoparticles with small size and narrow size distribution from relative large and agglomerated transition-metal particles dispersed in [Bmim][PF6] by simple laser irradiation. In our preliminary studies, the ionic liquids was applied as the solvents of gamma radiation polymerization of different monomers[6-8] and synthesized very stable Au nanoparticles in [Me3NC2H4OH]+[ZnCl3]- by gamma- radiation[9].
In addition, RTILs are widely studied in nuclear fuel cycle processing, and several patents have been issued. A simple study of the effect of α, β, γ irradiations on 1,3-alkylmethylimidazolium ionic liquids possessing nitrate or chloride anion showed that less than 1% of RTILs underwent radiolysis when exposed to 400 kGy and the stability of ionic liquid was close to that of benzene[10].
Viscosity is one of the important properties of ionic liquid, and also depends on temperature. However, when ionic liquid is irradiated by gamma rays, some changes on its viscosity may occur. There is few information available on the rheological properties of ionic liquids submitted to gamma radiation process, and for this reason, rheological properties of gamma irradiated [Bmim][BF4] and [Bmim][PF6] were investigated in our previous study[11]. In this paper, [Me3NC2H4OH]+- [Zn2Cl5]- and [Me3NC2H4OH]+[Zn3Cl7]- we exposed to γ-ray for several irradiation doses, such as 0, 10, 20, 50, 100 kGy. And viscosities were measured at different shear rates and temperatures before and after irradiation. The current work in this paper aimed to verify the rheological properties of ionic liquids when submitted to gamma irradiation.
2 Experimental section
2.1 Preparation of RTIL
ZnCl2 and choline chloride [Me3NC2H4OH]Cl with a purity of 99% were both obtained from America. Quaternary ammonium based ionic liquid [Me3NC2H4OH]+[Zn2Cl5]- and [Me3NC2H4OH]+[Zn3Cl7]- were prepared by heating mixtures of choline chloride and ZnCl2 in molar ratio of 1?2, and 1?3 according to the method in Ref.[12], respectively. The liquids obtained were colourless, with a freezing point between ca. 25 ℃ (1?2) and 45 ℃ (1?3). Then they were kept in vacuum at 100 ℃ overnight to remove the moisture and volatile impurities before use. All chemicals used were of analytical grade.
2.2 Irradiation
Irradiations were performed in 60Co (Shanghai Institute of Applied Physics, Chinese Academy of Sciences). The samples were irradiated in closed flasks, in nitrogen atmosphere and at room temperature. RTILs were saturated with N2 (nitrogen) in advance to prevent the oxidation of ionic liquids. Samples sealed in glass ampoules were exposed to total doses of 0, 10, 20, 50, 100 kGy and at the same dose rate of 10.9 Gy/min.
2.3 Rheological measurements
Rheological measurements were carried out on the advanced Rheometric expanded system (ARES, TA instrument). Steady shear viscosities were measured at different temperatures for [Me3NC2H4OH]+[Zn2Cl5]-, namely 25, 35 , 45 ℃; for [Me3NC2H4OH]+[Zn3Cl7]-, 45, 55, 65 ℃) and shear rates beween 0.1 and 25.1 s-1 by the parallel plate(25 mm in diameter) geometry. The temperature sweep of [Me3NC2H4OH]+[Zn2Cl5]- was performed in the linear viscoelastic regime of sample with temperature varied between 25 and 60 ℃, while [Me3NC2H4OH]+[Zn3Cl7]- with temperature varied between 45 and 70 ℃. Each measurement was carried after stabilization for 15 min. All measurements were performed at least in duplicate and the temperature deviation was ±0.2 ℃.
3 Results and discussion
3.1 Effect of shear rate
The effect of shear rate (the velocity gradient within the flowing liquid) on shear stress or viscosity indicates whether a liquid is Newtonian or non-Newtonian. Newtonian liquids display a viscosity which is independent of the shear rate. Pseudo-plastic behavior can be described by the Power Law model
![]() (1)
(1)
or
![]() (2)
(2)
where σ is the shear stress, Pa; ![]() is the shear rate, s-1; η is the apparent viscosity, Pa?s; k is the consistency coefficient and n is the flow behavior index. k and n are derived from the double logarithmic curve of the shear stress (or viscosity)—shear rate relationship[13].
is the shear rate, s-1; η is the apparent viscosity, Pa?s; k is the consistency coefficient and n is the flow behavior index. k and n are derived from the double logarithmic curve of the shear stress (or viscosity)—shear rate relationship[13].
The viscosities of ionic liquids (unirradiated and irradiated) were measured at shear rates 0.25-25.10 s-1 and three temperatures. n derived from logarithmic curve of the shear stress against shear rate appropriates to 1, indicating Newtonian behavior. For [Me3NC2H4OH]+- [Zn2Cl5]- between 25 ℃ and 45 ℃, [Me3NC2H4OH]+- [Zn3Cl7]- between 45 ℃ and 65℃, the viscosity values at different shear rates are constant within experimental error. For clarity, the shear stress was plotted against shear rate for 20 kGy irradiated [Me3NC2H4OH]+- [Zn2Cl5]- and [Me3NC2H4OH]+[Zn3Cl7]- in three temperatures in Fig.1(a) and Fig.2(a) respectively, and the according viscosity measurements against shear rate are plotted in Fig.1(b) and Fig.2(b), respectively. The viscosity values remain almost constant as the shear rate varies, confirming the Newtonian behavior. The averages and standard deviation of viscosities submitted to a statistical treatment are plotted in Tables 1 and 2.
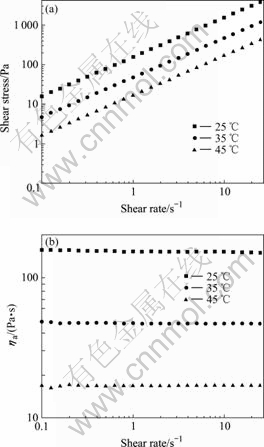
Fig.1 Shear stress vs shear rate (a), apparent viscosity vs shear rate (b) for 20 kGy irradiated [Me3NC2H4OH]+[Zn2Cl5]- at different temperatures
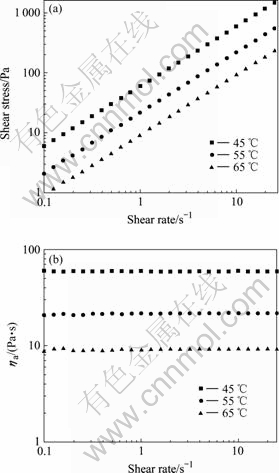
Fig.2 Shear stress vs shear rate (a), apparent viscosity vs shear rate (b) for 20 kGy irradiated [Me3NC2H4OH]+[Zn3Cl7]- at different temperatures
Table 1 Averages and standard deviation of viscosity of [Me3NC2H4OH]+[Zn2Cl5]- in functions of different irradiation doses and temperatures Viscosity/(Pa?s)

Table 2 Averages and standard deviation of viscosity values of [Me3NC2H4OH]+[Zn3Cl7]- in functions of different irradiation doses and temperatures Viscosity/(Pa?s)

3.2 Effect of temperature
Tables 1 and 2 list the effects of temperature on the viscosity of quaternary ammonium based ionic liquids. It is observed that mean values in the same column are significantly different, the viscosities decrease with the increase of temperature. Fig.3 shows the curve of viscosity as functions of temperature and irradiation doses. These data can be fitted by the Arrhenius equation:
ηa=Kexp[Eη/(RT)] (3)
where ηa is apparent viscosity, Pa?s; K is material constant; Eη is the activation energy for viscous flow, J/mol; R is the gas constant, 8.314 J/(mol?K); T is the absolute temperature, K. The activation energy for viscous flow (Eη) and K were calculated from the slope and intercept of the Arrhenius plot, respectively. The activation energy for viscous flow (Eη) for unirradiated and irradiated [Me3NC2H4OH]+[Zn2Cl5]- and [Me3NC2H4OH]+[Zn3Cl7]- are plotted in Table 3. Eη varies a little as the irradiation dose varies.

Fig.3 Plots of viscosity as functions of temperature and irradiation doses: (a) [Me3NC2H4OH]+[Zn2Cl5]-, 298.15-333.15 K; (b) [Me3NC2H4OH]+[Zn3Cl7]-, 318.15-343.15 K
3.3 Effect of irradiation dose
As shown in Table 1, the mean viscosities of [Me3NC2H4OH]+[Zn2Cl5]- is almost independent of the irradiation dose except 20 kGy irradiated samples, that is to say, gamma radiation does not impair the viscosity;
Table 3 Activation energy for viscous flow for [Me3NC2H4OH]+[Zn2Cl5]- and [Me3NC2H4OH]+[Zn3Cl7]- under different irradiation doses Eη/(kJ?mol-1)

While the mean viscosity of irradiated [Me3NC2H4OH]+ [Zn3Cl7]- is lower than that of the unirradiated sample by a factor of 10% in Table 2, but the viscosity of irradiated [Me3NC2H4OH]+[Zn3Cl7]- is independent of radiation dose. Their difference towards radiation may be due to different kinds of anions.
4 Conclusions
1) The rheological properties of unirradiated and irradiated [Me3NC2H4OH]+[Zn2Cl5]- and [Me3NC2H4- OH]+[Zn3Cl7]- are measured at different shear rates (0.1- 25.1 s-1) and three temperatures.
2) All samples show a Newtonian behavior and gamma radiation does not affect it. Viscosities decrease with the increase of temperature and follow an Arrhenius expression.
3) Gamma radiation does not decrease the viscosity of [Me3NC2H4OH]+[Zn2Cl5]- (within experimental error) except 20 kGy irradiated samples. But it decreases the viscosity of [Me3NC2H4OH]+[Zn3Cl7]- by 10%.
4) The viscosity of irradiated [Me3NC2H4OH]+- [Zn3Cl7]- is independent of radiation dose. It is concluded that the radiation stability of [Me3NC2H4- OH]+[Zn2Cl5]- is higher than that of [Me3NC2H4OH]+- [Zn3Cl7]-.
References
[1] WELTON T. Room-Temperature ionic liquids-solvents for synthesis and catalysis [J]. J Chem Rev, 1999, 99: 2071-2083.
[2] EARLE M J, SEDDON K R. Ionic liquids-green solvents for the future [J]. J Pure Appl Chem, 2000, 72: 1391-1398.
[3] WASSERSCHEID P, KEIM W. Ionic liquids—New “solutions” for transition metal catalysis [J]. J Angew Chem Int Ed, 2000, 39: 3772-3789.
[4] YANG Q, DIONYSIOU D. Photolytic degradation of chlorinated phenols in room temperature ionic liquids [J]. J Photochem Photobiol Part A: Chem, 2004, 165: 229-240.
[5] GELESKY M A, UMPIERRE A P, MACHADO G. Laser-Induced fragmentation of transition metal nanoparticles in ionic liquids [J]. J Am Chem Soc, 2005, 127: 4588-4589.
[6] WU Guo-zhong, LIU Yao-dong, LONG De-wu. Effects of ionic liquid [Me3NC2H4OH]+[ZnCl3]- on γ-radiation polymerization of methyl methacrylate in ethanol and N, N-dimethylformamide [J]. Macromol, Rapid Commun, 2005, 26: 57-61.
[7] LIU Yao-dong, WU Guo-zhong. On the mechanism of radiation-induced polymerization of vinyl monomers in ionic liquid [J]. Radiation Physics and Chemistry, 2005, 73: 159-162.
[8] LIU Yao-dong, WU Guo-zhong, LONG De-wu, QI Min-ying, ZHU Zhi-yong. 60Co γ-irradiation initiated polymerization in ionic liquids—The effect of carbon-chain length of monomer [J]. Nuclear Instruments and Methods in Physics Research B, 2005, 236(1/4): 443-448.
[9] CHEN S M, WU G Z, LIU Y D. Stabilized and size-tunable gold nanoparticles formed in a quaternary ammonium-based room-temperature ionic liquid under γ-irradiation [J]. Nanotechnology, 2005, 16: 1-5.
[10] ALLEN D, BASTON G, BRADLEY A E, GORMAN T, HAILE A, HAMBLETT I, et al. An investigation of the radiochemical stability of ionic liquids [J]. Green Chem, 2002, 4: 152-158.
[11] QI Ming-ying, WU Guo-zhong, LI Qian-mei, LUO Ying-she. γ-radiation effect on ionic liquid [bmim][BF4] [J]. Radiation Physics and Chemistry, 2008, 77: 877-883.
[12] ABBOTT A P, CAPPER G, et al. Preparation of novel, moisture-stable, Lewis-acidic ionic liquids containing quaternary ammonium salts with functional side chains [C]// Chem Commun. 2001: 2010-2011.
[13] LI Qian-mei, WU Guo-zhong, LIU Yao-dong, LUO Ying-she. A rheological study of binary mixtures of ionic liquid [Me3NC2H4OH]+[Zn2Cl5]- and ethanol [J]. Appl Rheol, 2006, 16: 334 -339.
Foundation item: Project(26120231) supported by the “Hundred Talents” project of the Chinese Academy of Science
Received date: 2008-06-25; Accepted date: 2008-08-05
Corresponding author: WU Guo-zhong, Professor; Tel: +86-21-59558905; E-mail: wuguozhong@sinap.ac.cn
- Rheological properties of gamma irradiated [Me3NC2H4OH]+[Zn2Cl5]- and [Me3NC2H4OH]+[Zn3Cl7]- ionic liquids


