Trans. Nonferrous Met. Soc. China 30(2020) 3103-3113
Scandium recovery from raffinate copper leach solution as potential new source with ion exchange method
H. HAJMOHAMMADI1, A. H. JAFARI1, M. ESKANDARI NASAB2
1. Department of Metallurgy and Materials Science Engineering, Shahid Bahonar University of Kerman, Iran;
2. Department of Mineral Engineering, Zarand High Education Complex, Zarand, Iran
Received 4 December 2019; accepted 14 September 2020
Abstract:
Raffinate copper leach solution of the Iran Sarcheshmeh copper complex has up to 3 mg/L scandium (Sc), which is significantly better than many existing sources, making it a possible source for the recovery of Sc using the ion exchange method. Visual Minteq software was employed to ascertain the ionic species likely to be formed under operational conditions in the mine and for selecting the suitable ion exchange resin. The cationic resin thus chosen was employed statically with ions-bearing synthesized solutions and statically/dynamically for actual copper mining raffinate solution. Room temperature and pH of 1.5 showed the highest Sc adsorption. The dynamic tests established the full saturation of the resin at 450 BV of the raffinate solution flow. Using sodium carbonate for elution, desorption of Sc, Y and Ce from the resin during static elution tests at constant duration was higher than that of Fe, Al and Cu. The results from the dynamic tests followed similar trends for the priority and the extent of the elution process. Desorption results from specimens of dynamic tests show a 60:1 concentration ratio leading to a 186 mg/L Sc-rich solution.
Key words:
scandium; ion exchange; sorption; desorption; copper leach solution;
1 Introduction
Scandium is one of the 17 rare earth elements [1], which due to its unique chemical and mechanical properties is considered one of the most promising candidates for the future energy and transport industry applications. Thus, it has already been classified as one of the metals considered to be strategic and critical for the future by the European Union [2]. It is widely used in light alloys and solid fuel cells, nuclear technology, special coatings, sodium-scandium lamps, solid electrolytes, high- temperature superconductors, crystal lasers, X-ray anodes, electronics and advanced ceramics [3-5].
Rare earth elements (REEs) are expensive in general due to their scarcity and difficult extraction and separation processes [6], and scandium is one of the most expensive REEs [7]. To illustrate the point, with the average crustal abundance of 22×10-6 [8], it is the 31st element in the earth’s crust. Besides, scandium-extractable ore at economic concentrations is very scarce [9], so it needs to be produced from by-products of industrial extraction of a range of other metals such as uranium, titanium, tungsten, aluminum, nickel, tantalum and niobium [8,10,11]. For example, the uranium leach solution having up to 1 mg/L of scandium is used in some Australian and US mines [8,12].
Generally, scandium extraction studies are focused on sources such as uranium leach, aluminum red mud, tungsten, nickel and zirconium production residues. Although studies of extracting rhenium, yttrium and neodymium from copper leach solution have been reported, so far no scientific research has been published on the extraction of scandium from such solutions. The leach solution of Iran’s Sarcheshmeh Copper Mine, has about 3 mg/L of prized scandium, about three times as in usual uranium leach solutions [13-15], without the radioactive hazards associated with uranium.
The extraction methods of rare earth elements fall into four main categories of solvent extraction, ionic liquids, solvent-impregnated resins and ion exchange [16-19]. The first three methods rest on the same chemo-physical principles and, are all variations of the solvent extraction technique which, has major environmental concerns as well as problems with evaporation of liquids during operation and also, high limit for minimum concentration of the ion to be extracted if the technique were to be used. The only difference between the first two techniques is some improvement in their extraction efficiency without addressing the above issues [4,20]. Both ionic liquids and the solvent-impregnated resin methods attempt to limit these deficiencies [16]; however, although improved in some respects, they suffer from the toxicity of their reagents, challenging preparation stages and above all [21]; complementary steps yet need to be developed before they are fully commercialized [22]. The ion exchange technique although not new has undergone enormous improvements while still being based on the reversible adsorption of ions at the solid/liquid interface [23]. The improvements are made in the increased ease of use and enhanced active surface to volume ratio for the maximum extraction capacity [24,25]. However, the main disadvantage ascribed to the ion exchange method is the lack of ion-specific resins for every rare earth metal that has led to the use of general-purpose anionic and cationic resins in experimentation with rare earth metal recovery [26]. Most electrolytes containing rare-earth ions are acidic in nature and cationic resins are the primary choice in such environments [15], although anionic resins are also reported for Sc-carrying oxalic acid solutions [27]. Dowex 50W-X8, Lewatit TP, Lewatit S 567, MTC 1600, Tulsion CH93, AmberliteTM IRN 77H, Purolite S95, Purolite 260, Purolite D5041 and Purolite C100, are all reported as testbeds for various ion separation regimes [15,28-30]. These resins and many more experimental polymers have shown different degrees of success but the Purolite C100 [17,29] and Dowex 50W-X8 [6,28] have a better track record in this field although none has been used for the Sc extraction from copper mining wastewater. Purolite C100 enjoys an advantage over Dowex 50W-X8 in adsorption capacity which is 2 eq/L compared to the 1.7 eq/L [26,31]. The pilot-scale use of Purolite C100 for rare earth metal separation has been reported but the other resins lack even such a stage of development [17]. These points plus relative price advantage and ease of availability made the present study focus on Purolite C100 as the main resin for testing against synthetic and actual mine leaching raffinate solutions.
Currently, large-scale sulfuric acid leaching for copper mining is operational in mines around the world, in which, a solution containing copper and low concentrations of scandium is obtained. The copper is separated from the solution by solvent extraction followed by electrowinning, but scandium remains in the raffinate solution, which can be used as a potential source for its recovery. Thus, this study is aimed to investigate the recovery of scandium from copper waste solution using ion exchange resin.
2 Experimental
2.1 Simulation of possible ion species
Metals in an aqueous solution can form anions or cations [12], therefore, a suitable anionic or cationic resin should be used for their optimal removal. Visual Minteq software version 3.1 was used to select the appropriate resin based on the modeling of chemical equilibrium feasible under the set conditions that could lead to the formation of various ionic species, the concentrations, the solubility of solid-phase equilibrium products and minerals in the copper raffinate leach solutions. This software has been utilized to study the type and concentration of ionic species during leaching of minerals and also sorption of elements by zeolite, various resins and in solvent extraction [32,33].
2.2 Reagents and chemicals
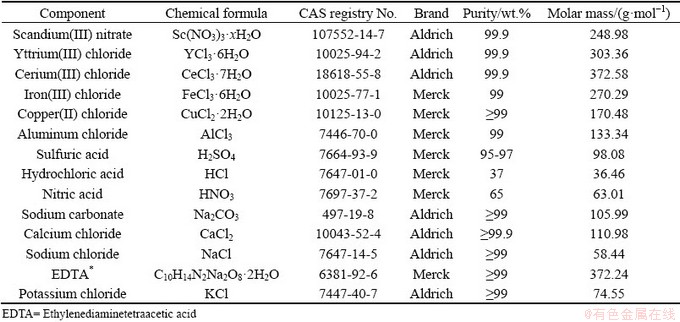
The chemicals used were analar grade as indicated in Table 1 which shows their chemical abstracts service (CAS) registry number, purity and molar mass. The metallic salts used for preparing synthetic solutions were in nitrate or chloride form. This is because the anion of metal salts does not play an important role in the ionic strength of the synthetic solutions which depend only on the total concentration and the charge carried by ions in the electrolyte [34] which is the same whether nitrate or chloride is used.
Table 1 CAS registry number, purity and molar mass of chemicals
Purolite C100Na ion exchange resin was also commercially available per the properties presented in Table 2. Figure 1 shows the chemical structure of this resin.
Table 2 Physical and chemical properties of Purolite C100Na ion exchange resin
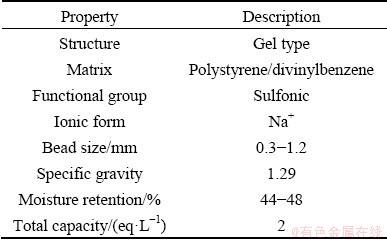
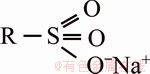
Fig. 1 Structure of Purolite C100Na ion exchange resin (R represents styrene-divinylbenzene copolymer)
2.3 Preparation of resin
The purpose of the resin preparation is to remove impurities and open up the resin pores and cavities for optimal sorption. The resin was submerged in a large beaker containing five times its volume deionized distilled water to be vigorously stirred by a shaker for 1 h, after which the liquid is left to settle. The water was then drained and the process was repeated several times before the mixture was filtered, and the resin was spread on glass and dried in an oven at 50 °C for 24 h. The dried resin was transferred to a desiccator for storage and later use.
2.4 Effect of solution acidity on ion sorption
The effect of electrolyte pH on scandium sorption was investigated, using a synthetic solution containing 10-3 mol/L each of scandium, yttrium, cerium, aluminum, iron and copper. The pH was altered between 0.13 and 2. Tests were performed using 0.1 g of washed and dried resin and 50 mL of synthetic solution continuously stirred in a shaker at 25 °C for a duration of 24 h. The resin was then extracted from the solution and analyzed by ICP-MS (Agilent Technologies HP4500), and the sorption (S) of elements was calculated as follows:
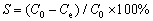 (1)
(1)
where C0 and Ce are the initial and equilibrium concentrations of scandium in the aqueous phase, respectively.
2.5 Effect of temperature on scandium ion sorption
The effect of electrolyte temperature on scandium sorption was investigated to find the optimum temperature. In these experiments similar set up as described above was used; however, the solution was kept at pH=1.5 while the temperatures were adjusted at 25, 35, 45 and 55 °C for 24 h and afterward the sorption was calculated.
2.6 Sorption isotherm test
Different adsorption isotherms were assessed to establish the most appropriate model for describing the adsorption of the two elements of scandium and its main rare earth competitor yttrium [6]. Isothermic experiments were performed for either of them separately on 50 mL of acid solution at pH=1.5 containing 150-400 mg/L of scandium while the yttrium concentrations were varied between 300 and 500 mg/L to simulate the range of concentrations encountered in real leach raffinate and actual mine solutions. All tests were conducted at 25 °C for 24 h while the electrolytes were exposed to 0.1 g of washed and dried resin. To determine the isotherm model governing the sorption process, equilibrium data from scandium and yttrium adsorption were analyzed using four generally applied isotherms: Langmuir, Freundlich, Temkin and Dubinin-Radushkevich (Eqs. (2)-(6)) [35-37]:
Langmuir: 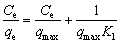 (2)
(2)
Freundlich: lg qe=lg Kf+(1/n)lg Ce (3)
Temkin: 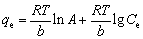 (4)
(4)
Dubinin-Radushkevich (D-R):
 (5)
(5)
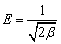 (6)
(6)
where qe is equilibrium capacity of the resin; Ce is equilibrium concentration of the metal ion in solution; qmax is the maximum resin capacity; Kl is the Langmuir constant related to the sorption energy; Kf is the Freundlich constant for the sorption capacity; 1/n is the heterogeneity parameter; A and b are Temkin constants; qm is the Dublin-Radiskovic capacity; β is the constant of energy; E is average sorption energy per mole of absorbent.
2.7 Scandium ion adsorption under dynamic state
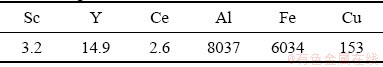
In dynamic sorption tests, the flow of the real raffinate from copper leach solutions at pH=1.5 whose composition is listed in Table 3 was passed through a column containing 20 mL of resin at 100 mL/h. The solution was then analyzed at intervals by ICP-MS for determining the concentration of various elements.
Table 3 Chemical composition of copper raffinate leach solution (mg/L)
2.8 Elution and desorption of scandium in static experiments
Hydrochloric acid, nitric acid, and EDTA laced hydrochloric acid, sodium carbonate, calcium chloride, sodium chloride and potassium chloride were used as the eluent for scandium desorption from this resin. Each elution test was carried out on 0.1 g of the dry resin following saturation during the adsorption tests. The resin was removed after submerging in the eluent under test and the solution was analyzed by ICP-MS to determine the desorption (d):
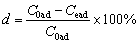 (7)
(7)
where C0ad and Cead are the initial and equilibrium concentrations of scandium in the sorbent, respectively.
2.9 Elution and desorption of scandium in dynamic experiments
The result of the static tests showed that the sodium carbonate solution has the highest desorption efficiency for scandium, yttrium and cerium, while the desorption of the intruding elements including iron, aluminum and copper is postponed to later stages. Therefore, this compound was selected for studying dynamic elution tests where the eluent would pass continuously through the column containing 20 mL of the saturated resin at a flow rate of 100 mL/h. Then, the passing solution was analyzed by ICP-MS to determine the concentration of the elements at intervals.
3 Results and discussion
3.1 Simulation of ion species
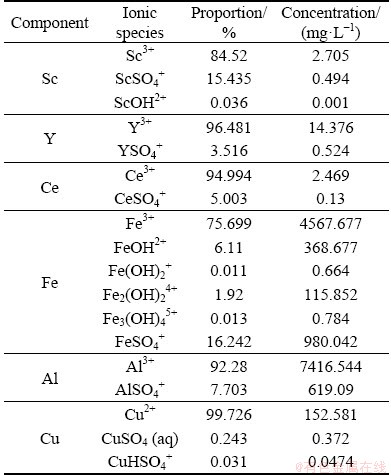
Past research indicates that scandium in sulfate media appears mainly as cationic species [15]; however, the choice of the most appropriate ion exchange resins for the extraction of metal ions depends on accurate prediction of the form and the abundance of the ionic species present in a solution under actual operating conditions [12]. Therefore, types of scandium and other elements present in ionic form were investigated by Visual Minteq software under the conditions of raffinate formed in copper leaching operation. As listed in Table 4, the raffinate solution forms three cationic species mainly 84.52% Sc3+ of the total followed by ScSO4+ and ScOH2+. This justifies the choice of Purolite 100Na as the polymeric resin for Sc adsorption.
Table 4 Simulation of type and percentage of ionic species of scandium, yttrium, cerium, iron, aluminum, and copper in raffinate copper leach solution using Visual Minteq software
3.2 Effect of acidity on sorption of various ionic species from sulfate solution
One of the controllable factors that influence the formation of ionic species and subsequent sorption of elements from a solution is pH [12]. This effect was studied on scandium sorption in the presence of yttrium, cerium, aluminum, iron and copper in the static tests. As shown in Fig. 2, increasing the medium pH enhances the sorption of scandium and other RE elements. Experimental results demonstrated that the sorption of scandium at pH≥1.5 reached the highest level at about 90%. Based on the pH of the actual copper raffinate solution, however, the pH=1.5 was preferred as there is no need to change the pH of the raffinate solution for peak scandium sorption while, iron sorption at this pH was about 20% lower.
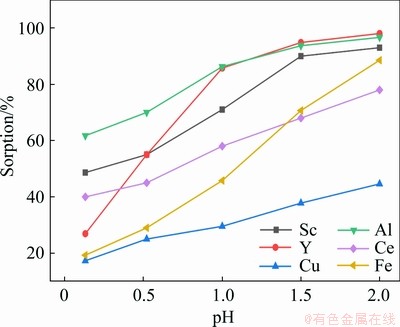
Fig. 2 Effect of medium pH on scandium sorption in the presence of yttrium, cerium, aluminum, iron and copper in static tests
3.3 Effect of temperature on sorption of various ions from sulfate solution
Another controllable factor that influences ionic species formation and sorption from solutions is the solution temperature [12]. This effect on scandium sorption in the presence of yttrium, cerium, aluminum, iron and copper was studied in static set up. As shown in Fig. 3, the sorption of all the elements, except iron, decreased as temperature increased. In the case of iron, the sorptions at 25 and 55 °C were about 70% and 80%, respectively. Therefore, the lower medium temperature is preferable as it combines the highest sorption of scandium with the minimum iron adsorption. On a purely practical level, one also has to take into account that any increase in temperature is associated with the added cost of energy, so 25 °C is optimum in this regard as well.

Fig. 3 Effect of medium temperature on scandium sorption in the presence of yttrium, cerium, aluminum, iron and copper in static set up tests
3.4 Various sorption isotherms for scandium and yttrium
Sorption isotherms are used to describe how the ions and molecules of the adsorbed material react with the sites on the sorbent surface if the temperature is kept constant, i.e. the equilibrium parameters predict that how the sorbent and adsorbed materials would mutually interact. Equilibrium studies can help for understanding the sorption process, optimizing the parameters affecting sorption, calculating the sorption capacity, and the general behavior of elements during the sorption [38,39]. The present study evaluated the constants of four isotherms commonly applied to the problem of ionic adsorption. These include Langmuir, Freundlich, Temkin and Dubinin- Radushkevich whose equations for two elements of scandium and its main competitor for adsorption, yttrium [6], were evaluated and their parameters were determined. Table 5 shows a high coefficient of determination (R2) for adaptation to the Freundlich model of adsorption data for both scandium and yttrium, indicating that it is the best model describing their sorption mechanism from aqueous solutions.
In the Freundlich model, when the value of the parameter (n) is in the range of 1-10, the sorption process is considered to be optimal. The higher values of n indicate increased heterogeneity of the sorbent surface for element sorption and, thus, enhanced the adsorption [40]. According to Table 5, the value of n for both elements is in this range, indicating optimal sorption from the solution; however, a slightly higher value for yttrium when examined individually may be taken as its tendency to get absorbed better. This is also shown in Figs. 2 and 3 from experiments at different pH and temperatures, but, this variation needs to be investigated specifically when the ions compete for the adsorbent sites.
Table 5 Isotherm constants of different sorption models for scandium and yttrium on Purolite C100Na resin
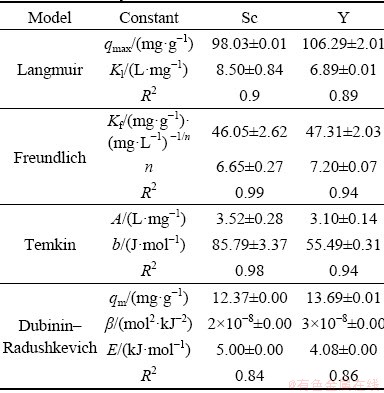
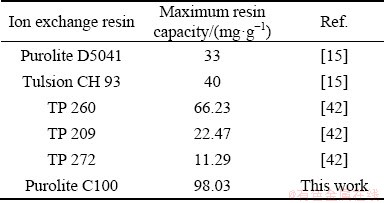
The Langmuir model is one of the earliest isotherms used to describe the sorption from aqueous solutions. It assumes no interactions between the adsorbed ions and the uniform distribution of active surface sites on the sorbent which leads to monolayer adsorption [41]. Table 5 shows the application of experimental results to the Langmuir model, indicating that the maximum monolayer capacities of Purolite resin for scandium and yttrium are 98.03 and 106.29 mg/g, respectively. This capacity is demonstrably high for Purolite resin when compared with the reported capacities of other resin adsorbents as can be seen in Table 6, signifying that it is the most appropriate for Sc extraction from aqueous solutions. An important point to consider is the Langmuir constant, Kl, which is used to gauge the strength of bonding formed during the sorption of an element. This as can be seen from data of Table 5 is higher for scandium than yttrium.
Table 6 Capacity for Sc adsorption with various ion exchange resins
The Temkin sorption isotherm was developed by considering the mutual absorbent/sorbent interactions at intermediate sorbent concentrations. This is based on the assumption that the heat of sorption, being a function of the temperature of all the molecules in the adsorbed layer, would decrease linearly as the process progresses and the adsorbent/ adsorbate interactions develop [43]. The Temkin specific constant (b) similar to the Kl value in the Langmuirian isotherm represents the amount of energy needed to separate a mole of adsorbed ion from the adsorbate, which again is higher for scandium than yttrium. Another isotherm used to describe the sorption mechanisms is the Dubinin– Radushkevich model [44]. Here, the constant (E) represents the average energy needed for desorption of the species adsorbed on the sorbent surface and has been used to determine the physical or chemical nature of the sorption process. It is argued that when the value of E is less than 8 kJ/mol, the sorption process is physical and when it falls in the 8-16 kJ/mol range, the adsorption is chemical [38]. As shown in Table 5, this value is less than 8 kJ/mol for both elements although slightly higher for scandium than yttrium, indicating the dominant role of physical sorption in both cases.
3.5 Sorption from raffinate solutions under dynamic experiments
The sorption curves of scandium, yttrium, cerium, aluminum, iron and copper by Purolite resin from the real solution of raffinate copper leach solutions are shown in Fig. 4.
As shown in Fig. 4, the complete resin saturation for scandium and yttrium happens at 450 and 300 BV flows of the actual solution, respectively.
3.6 Different eluents for desorption of cations from purolite in static experiments
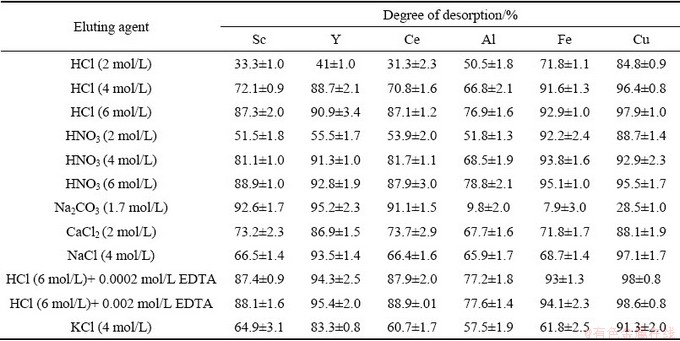
Studies for evaluating the desorption efficiency of various reagents have been reported for different ion-exchange resins and various cations. For example, HCl (2-6 mol/L) for DOWEX50W-X8 and a synthesized acid resin with a glycolamic acid function group, HNO3 (2-6 mol/L) for Purolite C100, Na2CO3 (1.7 mol/L) for TULSSION H 93, CaCl2 (2 mol/L) for Purolite S957 and NaCl (4 mol/L) for Purolite S957 are used for elution of rare earth elements scavenged by ion exchange resins [4,6,15,17,27]. EDTA has also been used as an adjunct complexing agent along with eluents for the desorption of rare earth elements [45]. The present work tested these most reported eluents for the specific task of desorbing various cations at various time intervals and assessed the observed efficiencies. HCl (2-6 mol/L), HNO3 (2-6 mol/L), Na2CO3 (1.7 mol/L), CaCl2 (2 mol/L), NaCl (4 mol/L) and EDTA added HCl solution were examined to assess it for efficiency as an eluent for scandium desorption and also to investigate the effect EDTA may have on the desorption of other ions present in the tested solutions.
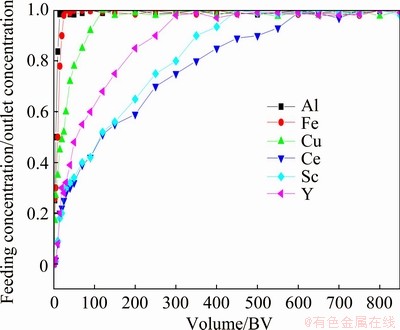
Fig. 4 Sorption curves on Purolite C100Na resin of cations present in raffinate copper leach solutions
The desorption of scandium and other elements from Purolite resin using the above reagents is shown in Table 7. The 1.7 mol/L sodium carbonate solution has the fastest and highest desorption for scandium, yttrium and cerium, and the lowest desorption for the intruding elements including iron, aluminum and copper. The EDTA results showed only a slight effect on the desorption of the above elements, so sodium carbonate (1.7 mol/L) was selected as the preferred eluent for the dynamic desorption phase.
Table 7 Degree of scandium desorption from saturated Purolite C100Na resin by various eluents
3.7 Elution and desorption studies using Na2CO3 (1.7 mol/L) in dynamic tests
As mentioned before, the 1.7 mol/L sodium carbonate used for desorption in static tests, showed high desorption efficiency for scandium, yttrium and cerium while deferring desorption for iron, aluminum and copper, so it was tested under dynamic conditions as well. The elution curves for this step are shown in Fig. 5. Fifteen separate samples were collected during the desorption stage with each sample representing a total volume of 1 BV or 20 mL of wash. The peak value of scandium appeared during desorption at 7 BV after yttrium peak. Earlier the importance of Kl value in the Langmuir model as the measure of sorption strength of a particular sorbent was noted. As shown in Table 5, the Kl was lower for yttrium than that for scandium, and thus, the precedence of yttrium over scandium during desorption, as can be seen in Fig. 5, is to be expected. In other words, the yttrium peak appears first due to its lower adhesion to the resin followed by the scandium peak with its strong adhesion. This is also confirmed by the calculated parameter b in the Temkin and E in the Dubinin–Radushkevich models, both of which represent the amount of energy required to separate a mole of the adsorbate. According to Table 5, both of these parameters are lower for yttrium than those for scandium; therefore, less energy is required for its separation from the resin.
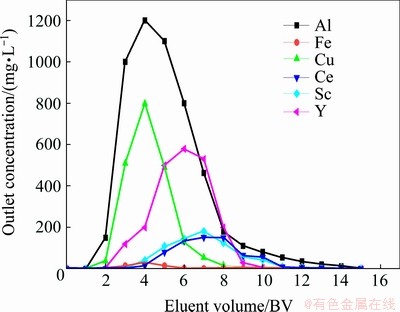
Fig. 5 Elution curves of certain elements from Purolite C100Na resin by Na2CO3 (1.7 mol/L) solution
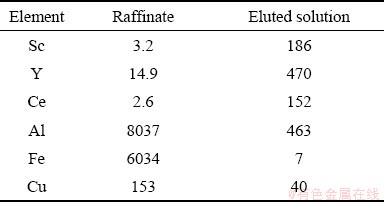
The raffinate solution left from leaching operation of the copper mine under investigation carries 3 mg/L of Sc and as Fig. 5 demonstrates that following the Sc extraction process the eluted liquid contains around 180 mg/L of Sc at 7 BV, showing a respectable 60:1 concentrating ratio. Table 8 lists the concentrations of Sc, Y, Ce, Al, Fe and Cu before and after the scavenging process, exhibiting that the first three elements have increased considerably in concentration while the latter three have diminished substantially. The result is a Sc-, Y-, Ce-rich solution, lending itself to any of the more economically appropriate separation techniques. This two-stage approach has been reported [6,17] for scavenging low-grade rare earth metals first through the ion exchange method followed by solvent extraction.
Table 8 Chemical composition sof raffinate and eluted solution at 7 BV (mg/L)
The recovery of rare earth metals by ion exchange resins may be done through scavenging the intruding elements such as iron and aluminum from the electrolyte first to be followed by the actual resin operation. For instance, Bayan Obo mine in China uses lime to precipitate iron and aluminum first [46]; however, in his study the recovery of Sc was initiated from raw raffinate solution directly received from the mining operation without a priori sequestration stage. This leaves avenues for further work with a two- or three-stage extraction where the final efficiency may be even high.
4 Conclusions
(1) Visual Minteq modeling indicated that Sc in the copper raffinate solution is present mainly in cationic form, therefore, recovering it by ion-exchange using Purolite C100Na was decided upon. This was done with variables of pH and temperature. Batch experiments showed that room temperature and pH 1.5 are the optimized conditions for adsorption.
(2) Modeling scandium and yttrium adsorption isotherms showed that it follows Freundlich type adsorption where the value of “n” affirms the suitability of the process.
(3) The desorption studies indicate that sodium carbonate has the highest elution for Sc, thus it was chosen as the selective eluent under dynamic tests.
(4) The outcome of experiments on the leaching solutions obtained from an actual mine indicated that a 60:1 ratio for Sc beneficiation in the final solution is routinely achievable.
References
[1] da COSTA T B, da SILVA M G C, VIEIRA MELISSA G A. Recovery of rare-earth metals from aqueous solutions by bio/adsorption using non-conventional materials: A review with recent studies and promising approaches in column applications [J]. Journal of Rare Earths, 2020, 38: 339-355.
[2] ALKAN G, YAGMURLU B, GRONEN L DITTRICH C, MA Yi-qian, STOPIC S, FRIEDRICH B. Selective silica gel free scandium extraction from iron-depleted red mud slags by dry digestion [J]. Hydrometallurgy, 2019, 185: 266-272.
[3] ZHANG Wei, YU Shu-qi, ZHANG Shi-chang, ZHOU Jie, NING Shun-yan, WANG Xin-peng, WEI Yue-zhou. Separation of scandium from the other rare earth elements with a novel macro-porous silica-polymer based adsorbent HDEHP/SiO2-P [J]. Hydrometallurgy, 2019, 185: 117-124.
[4] van NGUYEN N, IIZUKA A, SHIBATA E, NAKAMURA T. Recovery of Scandium from chloride media using the novel ion exchange resin [C]//Proceedings of the World Congress on Mechanical, Chemical, and Material Engineering. Barcelona, Spain, 2015: 3381-3384.
[5] ZHOU Hua-lei, LI Dong-yan, TIAN Ya-jun, CHEN Yun-fa. Extraction of scandium from red mud by modified activated carbon and kinetics study [J]. Rare Metals, 2008, 27: 223-227.
[6] OCHSENKUHN-PETROPULU M, LYBEROPULU T, PARISSAKIS G. Selective separation and determination of scandium from yttrium and lanthanides in red mud by a combined ion exchange/solvent extraction method [J]. Analytica Chimica Acta, 1995, 315: 231-237.
[7] AKCIL A, AKHMADIYEVA N, ABDULVALIYEV R, ABHILASH, MESHRAM P. Overview on extraction and separation of rare earth elements from red mud: Focus on scandium [J]. Mineral Processing and Extractive Metallurgy Review, 2018, 39: 145-151.
[8] WANG Wei-wei, PRANOLO Y, CHENG C Y. Metallurgical processes for scandium recovery from various resources: A review [J]. Hydrometallurgy, 2011, 108: 100-108.
[9] ZHANG Na, LI Hong-xu, LIU Xiao-ming. Recovery of scandium from bauxite residue-red mud: A review [J]. Rare Metals, 2016, 35: 887-900.
[10] WU Sheng-xi, ZHAO Long-sheng, WANG Liang-shi, HUANG Xiao-wei, DONG Jin-shi, FENG Zong-yu, CUI Da-li, ZHANG Li-feng. Dissolution behaviors of rare earth elements in phosphoric acid solutions [J]. Transactions of Nonferrous Metals Society of China, 2018, 28: 2375-2382.
[11] YU Qing, NING Shun-yan, ZHANG Wei, WANG Xin-peng, WEI Yue-zhou. Recovery of scandium from sulfuric acid solution with a macro porous TRPO/SiO2-P adsorbent [J]. Hydrometallurgy, 2018, 181: 74-81.
[12] HABASHI F. A textbook of hydrometallurgy [M]. 2nd ed. Quebec: Métallurgie Extractive, 1993.
[13] HISKEY J B, COPP R G. Recovery of yttrium and neodymium from copper pregnant leach solutions by solvent extraction [J]. Hydrometallurgy, 2018, 177: 21-26.
[14] NEBEKER N, HISKEY J B. Recovery of rhenium from copper leach solution by ion exchange [J]. Hydrometallurgy, 2012, 125-126: 64-68.
[15] SMIRNOV A L, TITOVA S M, RYCHKOV V N, BUNKOV G M, SEMENISHCHEV V S, KIRILLOV E, POPONIN N N, SVIRSKY I A. Study of scandium and thorium sorption from uranium leach liquors [J]. Journal of Radioanalytical and Nuclear Chemistry, 2017, 312: 277-283.
[16] DEVI N, SUKLA L B. Studies on liquid-liquid extraction of yttrium and separation from other rare earth elements using bifunctional ionic liquids [J]. Mineral Processing and Extractive Metallurgy Review, 2019, 40: 46-55.
[17] OCHSENKUHN-PETROPOULOU M T, HATZILYBERIS K S, MENDRINOS L N, SALMAS C E. Pilot-plant investigation of the leaching process for the recovery of scandium from red mud [J]. Industrial & Engineering Chemistry Research, 2002, 41: 5794-5801.
[18] LIAO Chun-fa, JIAO Yun-fen, LIANG Yong, JIANG Ping-guo, NIE Hua-ping. Adsorption-extraction mechanism of heavy rare earth by Cyanex272-P507 impregnated resin [J]. Transactions of Nonferrous Metals Society of China, 2010, 20: 1511-1516.
[19] WU Fang, XU Sheng-ming, LI Lin-yan, CHEN Song-zhe, XU Gang, XU Jing-ming. Recovery of valuable metals from anode material of hydrogen-nickel battery [J]. Transactions of Nonferrous Metals Society of China, 2009, 19: 468-473.
[20] OGATA T, NARITA H, TANAKA M. Adsorption behavior of rare earth elements on silica gel modified with diglycol amic acid [J]. Hydrometallurgy, 2015, 152: 178-182.
[21] HAN D, ROW K H. Recent applications of ionic liquids in separation technology [J]. Molecules, 2010, 15: 2405-2426.
[22] SHARAF M, YOSHIDA W, KUBOTA F, GOTO M. A novel binary-extractant-impregnated resin for selective recovery of scandium [J]. Journal of Chemical Engineering of Japan, 2019, 52: 49-55.
[23] KUMAR S, JAIN S. History, introduction, and kinetics of ion exchange materials [J]. Journal of Chemistry, 2013, 2013: 1-13.
[24] PAGE M J, SOLDENHOFF K, OGDEN M D. Comparative study of the application of chelating resins for rare earth recovery [J]. Hydrometallurgy, 2017, 169: 275-281.
[25] FU Yun-feng, XIAO Qing-gui, GAO Yi-ying, NING Peng-ge, XU Hong-bin, ZHANG Yi. Direct extraction of Mo(VI) from acidic leach solution of molybdenite ore by ion exchange resin: Batch and column adsorption studies [J]. Transactions of Nonferrous Metals Society of China, 2018, 28: 1660-1669.
[26] FELIPE E C B, BATISTA K A, LADERIA A C Q. Recovery of rare earth elements from acid mine drainage by ion exchange [J]. Environmental Technology, 2020: 1-32.
[27] WALTER R I. Anion exchange studies of Sc (III) and V (IV). Separation of scandium, titanium and vanadium [J]. Journal of Inorganic and Nuclear Chemistry, 1958, 6: 58-62.
[28] GHAZALA R A. Recovery of rare earth elements from uranium concentrate by using cation exchange resin [J]. Isotope and Radiation Research, 2015, 47: 219-230.
[29] RYCHKOV V N, KIRILLOV E V, KIRILLOV S V, BUNKOV G M, MASHKOVTSEV M A, BOTALOV M S, SEMENISHCHEV V S, VOLKOVICH V A. Selective ion exchange recovery of rare earth elements from uranium mining solutions [C]//AIP Conference Proceedings, 2016: 1-7.
[30] CHOUR Z, LAUBIE B, MOREL J L, TANG Y T, QIU R L, SIMONNOT M O, MUHR L. Recovery of rare earth elements from dicranopteris dichotoma by an enhanced ion exchange leaching process [J]. Chemical Engineering and Processing—Process Intensification, 2018, 130: 208-213.
[31] FOSTER J T T, HU Y, BOYER T H. Affinity of potassium-form cation exchange resin for alkaline earth and transition metals [J]. Separation and Purification Technology, 2017, 175: 229-237.
[32] FERNANDEZ-OLMO I, FERNANDEZ J L, IRABIEN A. Removal of arsenic (III), chromium (III) and iron (III) traces from hydrofluoric acid solutions by specialty anion exchangers [J]. Solvent Extraction and Ion Exchange, 2009, 27: 727-744.
[33] MERRIKHPOUR H, JALALI M. Comparative and competitive adsorption of cadmium, copper, nickel, and lead ions by Iranian natural zeolite [J]. Clean Technologies and Environmental Policy, 2013, 15: 303-316.
[34] RYDBERG J. Solvent extraction principles and practice: Revised and expanded [M]. 2nd ed. Florida, USA: CRC Press, Taylor & Francis Group, 2004.
[35] ZHU Xian-zheng, HUO Guang-sheng, NI Jie, SONG Qiong. Removal of tungsten and vanadium from molybdate solutions using ion exchange resin [J]. Transactions of Nonferrous Metals Society of China, 2017, 27: 2727-2732.
[36] MOGHIMI1 F, JAFARI A H, YOOZBASHIZADEH H, ASKARI M. Adsorption behavior of Sb(III) in single and binary Sb(III)-Fe(II) systems on cationic ion exchange resin: Adsorption equilibrium, kinetic and thermodynamic aspects [J]. Transactions of Nonferrous Metals Society of China, 2020, 30: 236-248.
[37] XIAO Yong, FENG Ning-ning, WANG Run-hua, DONG Hai-gang, GUO Zi-wen, CUI Hao, WU Hai-yan, LIU Xin-xing, XIE Jian-ping. Application of modified sepiolite as reusable adsorbent for Pd(II) sorption from acidic solutions [J]. Transactions of Nonferrous Metals Society of China, 2020, 30: 1375-1386.
[38] FATHI M B, REZAI B, KESHAVARZ ALAMDARI E, ALORRO R D. Equilibrium modeling in adsorption of Re and Mo ions from single and binary aqueous solutions on Dowex 21K resin [J]. Geosystem Engineering, 2018, 21: 73-80.
[39] WANG Cai-xia, HU Hui-ping, QIU Xue-jing, CHENG Ze-ying, MENG Lu-jia, ZHU Li. Synthesis of novel silica-supported chelating resin containing tert-butyl 2-picolyamino-N-acetate and its properties for selective adsorption of copper from simulated nickel electrolyte [J]. Transactions of Nonferrous Metals Society of China, 2018, 28: 2553-2565.
[40] SHAHMOHAMMADI-KALALAGH S. Isotherm and kinetic studies on adsorption of Pb, Zn and Cu by kaolinite [J]. Caspian Journal of Environmental Sciences, 2011, 9: 243-255.
[41] JING Qing-xiu, CHAI1 Li-yuan, HUANG Xiao-dong, TANG Chong-jian, GUO Huan, WANG Wei. Behavior of ammonium adsorption by clay mineral halloysite [J]. Transactions of Nonferrous Metals Society of China, 2017, 27: 1627-1635.
[42] BAO S X, HAWKER W, VAUGHAN J. Scandium loading on chelating and solvent impregnated resin from sulfate solution [J]. Solvent Extraction and Ion Exchange, 2018, 36: 100-113.
[43] DADA A O, OLALEKAN A P, OLATUNYA A M, DADA O. Langmuir, Freundlich, Temkin and Dubinin–Radushkevich isotherms studies of equilibrium sorption of Zn2+ onto phosphoric acid modified rice husk [J]. IOSR Journal of Applied Chemistry, 2012, 3: 38-45.
[44] GUNAY A, ARSLANKAYA E, TOSUN I. Lead removal from aqueous solution by natural and pretreated clinoptilolite: Adsorption equilibrium and kinetics [J]. Journal of Hazardous Materials, 2007, 146: 362-371.
[45] SEVENICH G J, FRITZ J S. Addition of complexing agents in ion chromatography for separation of polyvalent metal ions [J]. Analytical Chemistry, 1983, 55: 12-16.
[46] DING Yin-gui, WANG Jing-song, WANG Guang, XUE Qing-guo. Innovative methodology for separating of rare earth and iron from Bayan Obo complex iron ore [J]. ISIJ International, 2012, 52: 1772-1777.
用离子交换法从潜在钪资源的铜浸出残液中回收钪
H. HAJMOHAMMADI1, A. H. JAFARI1, M. ESKANDARI NASAB2
1. Department of Metallurgy and Materials Science Engineering, Shahid Bahonar University of Kerman, Iran;
2. Department of Mineral Engineering, Zarand High Education Complex, Zarand, Iran
摘 要:伊朗Sarcheshmeh铜联合企业的铜浸出残液含有高达3 mg/L的钪,明显高于许多现有的钪资源,因而成为用离子交换法回收钪的可能原料。使用Visual Minteq软件确定在矿山工艺条件下可能形成的离子物种,从而选择合适的离子交换树脂。所选择的阳离子树脂在静态试验中用于含有离子的合成溶液,以及在静态/动态试验中用于实际的铜矿浸出残液。室温和pH值为1.5时,钪的吸附率最高。在动态试验中,残液流量为450 BV时,树脂达到完全饱和。在恒定时间的静态洗脱试验中,使用碳酸钠洗脱,树脂中Sc、Y、Ce的解吸率高于Fe、Al和Cu。动态洗脱试验结果表明,洗脱的优先顺序和洗脱程度具有相似的趋势。动态试验样品的解吸结果表明,浓缩比达到60:1,可得到186 mg/L的富钪溶液。
关键词:钪;离子交换;吸附;解吸;铜浸出液
(Edited by Xiang-qun LI)
Corresponding author: A. H. JAFARI; Tel/Fax: +98-342817708; E-mail: Jafari.a.h@uk.ac.ir
DOI: 10.1016/S1003-6326(20)65446-2
Abstract: Raffinate copper leach solution of the Iran Sarcheshmeh copper complex has up to 3 mg/L scandium (Sc), which is significantly better than many existing sources, making it a possible source for the recovery of Sc using the ion exchange method. Visual Minteq software was employed to ascertain the ionic species likely to be formed under operational conditions in the mine and for selecting the suitable ion exchange resin. The cationic resin thus chosen was employed statically with ions-bearing synthesized solutions and statically/dynamically for actual copper mining raffinate solution. Room temperature and pH of 1.5 showed the highest Sc adsorption. The dynamic tests established the full saturation of the resin at 450 BV of the raffinate solution flow. Using sodium carbonate for elution, desorption of Sc, Y and Ce from the resin during static elution tests at constant duration was higher than that of Fe, Al and Cu. The results from the dynamic tests followed similar trends for the priority and the extent of the elution process. Desorption results from specimens of dynamic tests show a 60:1 concentration ratio leading to a 186 mg/L Sc-rich solution.


