Trans. Nonferrous Met. Soc. China 25(2015) 1551-1558
Microstructure and properties of Cu-2.3Fe-0.03P alloy during thermomechanical treatments
Qi-yi DONG1, Lei-nuo SHEN1, Ming-pu WANG1, Yan-lin JIA1,2, Zhou LI 1, Feng CAO1, Chang CHEN2
1. School of Materials Science and Engineering, Central South University, Changsha 410083, China;
2. School of Metallurgy and Environment, Central South University, Changsha 410083, China
Received 1 March 2014; accepted 3 March 2015
Abstract:
A short process without solution treatment was developed to manufacture Cu-2.3Fe-0.03P alloy strips. After hot rolling-quenching and cold rolling with 80% reduction, the alloy exhibited excellent resistance to recrystallization softening. The hardness and electrical conductivity of Cu-Fe-P alloy under different thermomechanical treatments were measured by hardness tester and double bridge tester, respectively, and the microstructure of the alloy was examined by optical microscopy and transmission electron microscopy. The results show that the finished product of Cu-2.3Fe-0.03P alloy was strengthened by work hardening, while the Fe precipitates with the size of about 25 nm stabilized the cold rolled structure. The conductivity decreased during cold rolling, especially for the pre-aged specimens, because the fine precipitates with the size smaller than 5 nm re-dissolved easily into the matrix. A Cu-Fe-P alloy with an electrical conductivity of 66% IACS and a hardness of HV 134 can be gained.
Key words:
Cu-Fe-P alloy; solution treatment; hot rolling; precipitation; recrystallization;
1 Introduction
A number of commercial Cu-based alloys have been developed, and the Cu-Fe system alloys are the most widely used in the lead frame market, due to their medium tensile strength, relatively better electrical conductivity and good softening resistance [1]. Although the mechanical properties of Cu-Fe-P alloys are not satisfactory, Cu-Fe-P alloys show excellent main properties for a lead frame material, such as 90° bend fatigue, 90° bend formability, corrosion-proof, solder ability and resistance of solder peeling off. In addition, Fe is a comparatively cheaper alloying element (compared with Cr, Ni, etc) in industry. Therefore, Cu-2.3Fe-0.03P (mass fraction, %) alloy (C19400) is a successful medium-strength and high-conductivity Cu alloy, and the general properties of the alloy are 62% IACS (International Annealed Copper Standard) in electrical conductivity, HV 150 in the hardness and 500 MPa in tensile strength [2].
It has been frequently reported that Cu-Fe-P alloys are strengthened by precipitation hardening and work hardening. However, unlike the Cu-Ni-Si [3] or Cu-Cr-Zr [4] system alloys, little or no age-hardening response can be found in the cold worked Cu-Fe-P alloy. The aging behaviors of Cu-2.3Fe-0.03P alloy are similar to those of Cu-Fe binary alloy. Small addition of P to Cu-Fe alloys usually forms Fe3P particles [5] which are considered to contribute direct effect on the nucleation of recrystallization [6]. KAZUHISA et al [6] found that through suitable thermomechanical treatments, the specimen with Fe particles (17 nm) and Fe3P particles (800 nm) had a high heat-resistance. NORIYUKI et al [1] pointed out that the heat-resistance property largely depended on the coherent Fe precipitates. While CAO et al [7] found that larger rigid Fe3P particles along the grain boundaries were much more effective in maintaining the stability of microstructure than smaller Fe particles. The reason for resistance to softening is not clear, further investigations are required.
An online hot rolling–quenching process was developed to manufacture Cu-Cr system alloys strips with high strength and electrical conductivity [4]. In the present study, the process was used to prepare Cu-2.3Fe-0.03P alloy, and the microstructure and properties of the alloy under different thermomechanical treatments were investigated. The mechanism of resistance to softening was also discussed.
2 Experimental
The experimental Cu-2.3Fe-0.03P alloy was prepared using electrolytic Cu, pure Fe, and Cu-13.6P (mass fraction, %) intermediate alloy in an intermediate frequency induction melting furnace, and cast in an ingot with a size of 150 mm × 70 mm × 35 mm.
After eliminating the surface defects, the ingot was homogenized at 960 °C for 6 h and then rapidly hot rolled (HR) to 4.4 mm, followed by quenching into cold water. Sheets cut from the HR plate were cold rolled (CR) with 80% reduction from 4.4 mm to 0.9 mm. For comparison, another sheet cut from the HR plate was solution treated (ST) at 900 °C for 1 h prior to cold rolling with the same deformation. Samples cut from the previous two kinds of CR sheets were subsequently isothermal aged (AG) at 450 °C in a salt-bath furnace for different time.
The Vickers hardness (HV) of the samples was measured at a load of 9.8 N and a holding time of 10 s. The related electrical conductivity was measured by a double bridge QJ-19 type machine at 20 °C. The conductivity values were converted to %IACS (International Annealed Copper Standard). Specimens were polished and etched with a solution of FeCl3 (5 g), HCl (25 mL) and H2O (100 mL), and then observed on a LEICA EC3 optical microscopy (OM). The rolled and aged OM samples were examined longitudinally. The transmission electron microscopy (TEM) specimens were mechanically polished and then jet electro-polished in a mixing solution of CH3OH and HNO3. The TEM observation was conducted by a TECNAI G220, with the operation voltage of 200 kV.
For comparison, four processes were selected in preparing Cu-Fe-P alloy, and they were provided as follows:
(A) HR+CR+AG at 450 °C;
(B) HR+ST+CR+AG at 450 °C;
(C) HR+pre-AG at 450 °C for 8 h+CR+AG;
(D) HR+ST+pre-AG at 450 °C for 8 h+CR+AG.
3 Results
3.1 Properties
Conventional thermomechanical treatments of Cu- based lead frame alloys consist of HR, ST, CR and AG. Figure 1 shows the variations of hardness and electrical conductivity of the hot rolled alloy with and without solution treatment. For the solution treated specimens, the hardness increased to HV 83 after aging at 450 °C for about 24 h, and then did not increase. The precipitation hardening effect was about HV 15, which was much less than that in the Cu-Ni-Si [3,8] or Cu-Cr [9,10] system alloys (about HV 100). However, for the non-solution treated specimens, the hardness was HV 98 in HR state, and was still HV 100 even after aging at 450 °C for 32 h, as if there was no response of age hardening.
Because of the solution heat treatment, the solute elements dissolved into the matrix, resulting in decreasing the electricity conductivity to 20.6% IACS. The curves of the electrical conductivity of the alloys aged at 450 °C are shown in Fig. 1(b). At the beginning of the aging, the electrical conductivity increased rapidly, since the concentration of the solute atoms decreased during aging. As aging time further increased, the alloy led to a slower rate of increase in the electrical conductivity. This result was consistent with the existing findings [11,12]. The conductivity of HR+ST alloy was 5% IACS lower than that of the HR alloy for a period of time. After aging at 450 °C for 32 h, the conductivity of hot rolled alloy increased up to 64% IACS, which was attributed to the precipitates dissolving out from the supersaturated solid solution. According to the current findings about hardness and electrical conductivity, the properties of HR alloy were superior to those of HR + ST alloy.

Fig. 1 Variation of hardness (a) and electrical conductivity (b) of hot rolled alloy with and without solution treatment aged at 450 °C
The isothermal aging curves of hardness and electrical conductivity during the four processes are shown in Fig. 2. The amounts of deformation in the four processes were identical. There was no sign of any age hardening, but the hardness decreased with aging time. However, the decrement of process A was much smaller than that of processes B and D. The hardness of HR+CR alloy reached to HV 146, along with an increase of HV 50 because of the work hardening effect. After aging at 450 °C for 16 h, the hardness dropped to HV 134. In comparison, the solution treated alloy showed a different aging curve. Process B showed a rapid over-aging response, and the hardness dropped to HV 88 after aging for only 30 min. The isothermal aging curves of electrical conductivity for the four processes have a similar tendency with that of the solution treated alloy. For process C, the hardness curve was intermediate between process A and process D. The initial electrical conductivity of process C was higher than other processes owing to the pre-aging, and this difference decreased with further aging.

Fig. 2 Variation of hardness (a) and electrical conductivity (b) of Cu-Fe-P alloy under four processes aged at 450 °C
Table 1 summarizes the typical hardness and electrical properties of Cu-Fe-P alloy under the four processes. Under adding the process of solution treatment, a significant decrease in the hardness and a slight difference in the electrical conductivity can be observed for the final state. The hardness of the alloy under process A was HV 136 after 8 h aging, which was HV 54 higher than that under process B. Furthermore, compared with the aged properties of the alloys under process C and process A, the added AG before CR slightly increased the conductivity but did not improve the hardness. The similar results under processes D and B were obtained. Therefore, the Cu-Fe-P alloy was dependent on CR for strengthening. In addition, CR decreased the electrical conductivity. The conductivity of CR alloy under processes C and D was about 9% IACS lower than that of HR(+ST)+AG alloy, which was attributed to the re-dissolution phenomenon of solute atoms.
3.2 Microstructure evolution
Figure 3 shows the optical micrographs of Cu-Fe-P alloy under different conditions. All samples were observed on longitudinal section. For the hot rolled alloy, the large grain particles of the as-cast state [13] were pressed and broken. The elongated grains of various sizes were parallel to the rolling direction, as shown in Fig. 3(a). Moreover, some small recrystallized grains were found at the boundaries. This result indicated that dynamic recrystallization occurred during the hot rolling. After solution treatment at 900 °C for 1 h, the microstructure mainly consisted of equiaxed grains with the size around 14 μm, as shown in Fig. 3(b).
Table 1 Hardness and electrical properties of Cu-Fe-P alloy under 4 processes

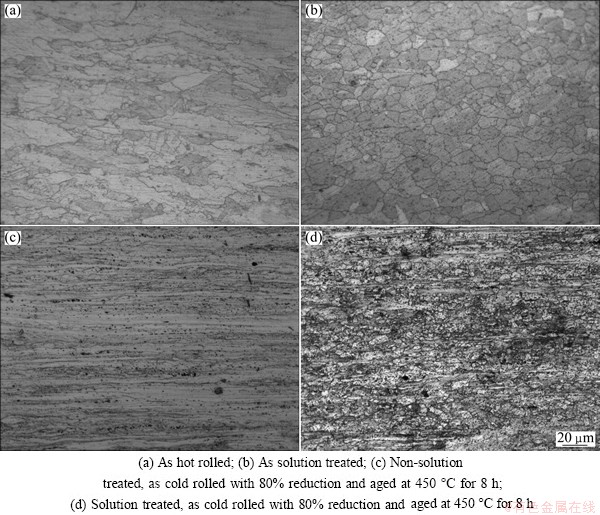
Fig. 3 Optical micrographs of Cu-Fe-P alloys under different conditions
Figures 3(c) and (d) show the optical microstructures of the aged alloy under process A and process B, respectively. Fibrous tissue was the main structure after subsequent cold rolling with 80% reduction. In the non-solution treated sample, the recrystallization did not occur after cold rolling and aging at 450 °C for 8 h; while in the solution treated sample (Processes B and D), only little evidence of the original fibrous tissue remained. These results supported the isothermal aging curves of the non-solution treated alloy, and the reduction in the hardness of alloy was due to the recrystallization softening.
3.3 Precipitation
Figure 4 shows the bright field images of the hot rolled Cu-Fe-P alloys and the corresponding selected area electron diffraction (SAED) pattern. High density of dislocations and cellular structures were observed in Fig. 4(a). The average size of the subgrains was about 1 μm. Figure 4(b) shows the high magnified image of the hot rolled structures. A large number of particles with a coffee-bean-shape contrast were observed. The occurrence of no-contrast lines in the particles were due to the coherence of precipitates and the matrix. The SAED pattern of Fig. 4(b) suggested that the no-contrast lines of particles were all perpendicular to the operating vector of 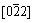 Cu. Pinned by the particles located in the gliding plane, dislocations can not glide freely. In different subgrains, the dislocation density is different. Figure 4(c) shows the high magnified image of the no-dislocation area by another operating vector of
Cu. Pinned by the particles located in the gliding plane, dislocations can not glide freely. In different subgrains, the dislocation density is different. Figure 4(c) shows the high magnified image of the no-dislocation area by another operating vector of 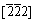 Cu. Obviously, the well dispersed particles are also evidenced by strain field contrast, and they may fall off during jet polishing thinning process (see the marked region A in Fig. 4(c)). From the dimension of the bright cavity such as region A, it can be estimated that the size of coherent particles is about 20 nm.
Cu. Obviously, the well dispersed particles are also evidenced by strain field contrast, and they may fall off during jet polishing thinning process (see the marked region A in Fig. 4(c)). From the dimension of the bright cavity such as region A, it can be estimated that the size of coherent particles is about 20 nm.
Figure 5 shows the bright field TEM images of the solution treated and non-solution treated samples after cold rolling and aging at different states, followed by water-cooling. For the solution treated samples, the dispersed second phase precipitated again from the matrix. When the solution treated samples were aged at 450 °C for 8 h, the dispersed precipitates with small size were observed in Fig. 5(a). While samples were aged at 500 °C for 64 h, these precipitates grew slowly up to about 10 nm (size even smaller because of strain contrast) (Fig. 5(b)). The corresponding SAED pattern was also shown in Fig. 5(b), and the electron beam axis of the SAED pattern was parallel to [112]Cu. Similar to Fig. 4(c), the no-contrast lines of particles were all perpendicular to the direction of  Cu. By comparison with the solution treated samples, another type of particles with size of 20-50 nm existed in the non-solution treated samples. When the aging temperature increased up to 500 °C, similar results on the size of the precipitates can be gained in Figs. 5(c) and (d).
Cu. By comparison with the solution treated samples, another type of particles with size of 20-50 nm existed in the non-solution treated samples. When the aging temperature increased up to 500 °C, similar results on the size of the precipitates can be gained in Figs. 5(c) and (d).

Fig. 4 Bright field images of hot rolled Cu-Fe-P alloy and SAED pattern, zone axis close to [011]Cu

Fig. 5 TEM images of solution treated samples (a, b) and non-solution treated samples (c,d) after cold rolling and aging at different conditions
4 Discussion
The Cu-Fe phase diagram shows that the solubility of Fe in Cu reduces from about 4% at 1094 °C to almost zero at room temperature. As the temperature decreases, the face-centered cubic (FCC) γ-Fe is precipitated from the Cu matrix. When the temperature is below 835 °C, the equilibrium precipitate is body-centered cubic (BCC) α-Fe. Previous studies revealed that the martensitic transformation γ-Fe→α-Fe in the Cu matrix was caused by the plastic deformation instead of the decreased temperature [14,15]. By the inquiry of Power Diffraction File (PDF), the lattice parameters of Cu, γ-Fe and α-Fe are 0.3615 nm (PDF 04-0836), 0.366 nm (PDF 52-0513) and 0.2866 nm (PDF 06-0696), respectively. The lattice mismatch value between Cu and γ-Fe is only 0.012. Owing to the excellent coherency possibilities between γ-Fe and Cu, the γ-Fe precipitates are revealed by strain-field contrast, as illustrated in Figs. 4(b) and 5(b). While the α-Fe precipitates are not perfectly coherent with the Cu matrix, they reveal as coarse spherical particles without contrast (as shown in Fig. 5(d)).
4.1 Effect of solution heat treatment
The hardness and electrical conductivity of HR+ST alloy were HV 68 and 20.6% IACS, respectively, and those of the non-solution treated alloy were HV 98 and 28% IACS, respectively. For the HR+ST alloy, the decline of the electrical conductivity indicates that some precipitates re-dissolve, which approves that the precipitates already exist after the process of hot rolling and quenching. The TEM image (Fig. 4(c)) thoroughly testified the conclusion. At the early stage of aging, the hardness of the hot rolled-quenched alloy fluctuated between HV 94 and HV 100, by the combined effects of precipitation hardening and recovery softening. The precipitation hardening of Cu-Fe-P alloy clearly was shown in the solution treated samples, which was enhanced by HV 15 after aging at 450 °C for 24 h.
The main purpose of solution heat treatment process is to get the uniform distribution of Fe particles and equiaxed grain. However, ST in the process B reflects a detrimental effect on the hardness of Cu-Fe-P specimens. The direct comparison of Figs. 3(c) and (d) apparently indicates that the recrystallization is the reason for the drastic hardness dropping. It is generally known that recrystallization will proceed by the migration of the new grain boundaries into the deformed matrix. It is suspected that the precipitates play an important role in hindering the migration of boundaries. For the solution treated samples, the γ-Fe particles in the hot rolling structure resolve into the matrix, the grain boundaries and the dislocations can glide freely without the pinning effect of precipitates, resulting in immediate recrystallization. While for the non-solution treated samples (Process A), the hardening due to cold work is maintained even after aging for 16 h.
A rapid increase in the conductivity of each process can be observed due to the precipitation of solute Fe element. As aging time increases, the accelerated rate of the electrical conductivity begins to decrease. Figure 2(b) shows that after aging for 8 h, the conductivity of cold rolled alloy is 63% IACS for process A, compared with 60.3% IACS for process B and 64% IACS for process C. For the samples solution treated at 900 °C, no drastic change in conductivity is shown under the final aging conditions.
4.2 Effect of cold rolling process
The γ-Fe particles presented in the hot rolling structures can not all transform to α-Fe because of two critical factors: 1) there is no dislocations cutting, and 2) the γ-Fe particle is smaller than 20 nm [14]. Thus, there are two kinds of precipitates in Fig. 5(c), which can be identified as small γ-Fe and large α-Fe. The γ-Fe particles with the size smaller than 5 nm precipitate from the matrix and grow slowly during aging. It is well known that the dispersed phases can stabilize the microstructure, but the precipitates aged at 450 °C are so fine that they easily dissolve into the Cu matrix during cold-rolling [1]. Other coarse spherical α-Fe particles observed in certain locations can fiercely pin the moving of grain boundaries [6], which will bring about the reservation of the fibrous structure. Although there is seemingly no response of any precipitation hardening, the process A avoids conventional solution treatment and reduces the manufacturing cost. Cu-2.3Fe-0.03P alloy with excellent resistance to softening was successfully developed. An electrical conductivity value of 66% IACS (Fig. 2(b)) and a hardness value of HV 134 (Fig. 2(a)) can be obtained after aging at 450 °C for 16 h (Process A).
It can be observed from Table 1 that a minor change in the electrical conductivity (3% IACS) occurs for the non-pre-aged and cold rolled samples. The phenomenon can be associated with the increasing density of dislocations induced by cold working, increasing the conducting electrons scattering and resulting in a reduction of conductivity of 1%-3% IACS [16]. The present work is in agreement with the prediction and the experimental results of Cu-Cr-Zr alloy reported by XIA et al [17]. Once the size of the precipitates is smaller than 5 nm, these fine precipitates are inclined to dissolve into the matrix during large cold deformation [1]. In Processes C and D, the conductivity decreased by 8.8% and 9.5% IACS during cold rolling, respectively, two thirds of the conductivity decreases due to the re-dissolution of fine Fe precipitates, while the rest of the decreases (3% IACS) resulted from the increases of conducting electrons scattering.
It has been reported that the strengthening of Cu-based alloys is mainly controlled by Orowan mechanism, and the precipitation hardening effect is dependent on the size and volume fraction of the precipitates [4]. The dislocations which were introduced by CR provide suitable sites for nucleation and are pinned by the Fe precipitates, resulting in the combination of work hardening and precipitation hardening. In addition, the purposes of adding the pre-aging treatment are to increase the electrical conductivity and to improve the strength by precipitation hardening, but no better result was obtained from adding the intermediate aging treatment for Cu-Fe-P final specimens. When the specimens were pre-aged at 450 °C for 8 h (Process D), those precipitates were too small and would re-dissolve during CR, so the volume fraction of the precipitates was limited. The increase of strength caused by precipitation hardening was not enough to make up for the decrease of strength caused by recrystallization softening, so the hardness fell obviously.
5 Conclusions
1) A short process without solution treatment was successfully developed to manufacture Cu-2.3Fe-0.03P alloy strips with excellent resistance to softening, which is due to the interaction between the Fe particles and dislocations. Either coherent or incoherent Fe particles with the size of about 25 nm can hinder recrystallization process.
2) For the pre-aged specimens, the conductivity decreases by 8.8%-9.5% IACS after cold rolling. Two thirds of the conductivity decreases due to the re-dissolution of fine precipitates with the size smaller than 5 nm, while the rest of the decreases resulted from the increases of conducting electrons scattering introduced by expanding dislocations.
3) The recrystallization process might cover the age hardening effect. The main strengthening mechanism of Cu-2.3Fe-0.03P alloy is work hardening. Cold rolling can produce high density of dislocations to cause work hardening, transform the bigger precipitates from initial γ-Fe to α-Fe, and re-dissolve the fine precipitates into the Cu matrix.
References
[1] NORIYUKI N, TONG C P, MAKOTO O, KATSUHIRO Y. A process for manufacturing Cu-Fe alloy C194-ESH with high electrical conductivity and excellent heat-resistance [J]. Hitachi Cable Review, 1999, 18: 61-66.
[2] LI Hua-qing, YANG Chun-xiu, XIANG Chao-jian, CAO Xing-min, GUO Fu-an, WANG Ming-pu, CHEN Wei. Processing technology for C194 alloy sheet and strip [J]. Transactions of Nonferrous Metals Society of China, 2007, 17: s1081-s1084.
[3] JIA Yan-lin, WANG Ming-pu, CHEN Chang, DONG Qi-yi, WANG Shan, LI Zhou. Orientation and diffraction patterns of δ-Ni2Si precipitates in Cu-Ni-Si alloy [J]. Journal of Alloys and Compounds, 2013, 557: 147-151.
[4] XIA Cheng-dong, ZHANG Wan, KANG Zhan-yuan, JIA Yan-lin, WU Yi-feng, ZHANG Ri, XU Gen-ying, WANG Ming-pu. High strength and high electrical conductivity Cu-Cr system alloys manufactured by hot rolling–quenching process and thermomechanical treatments [J]. Material Science and Engineering A, 2012, 538: 295-301.
[5] KIM H G, LEE T W, HAN S Z, EUH K, KIM W Y, LIM S H. Microstructural study on effects of C-alloying on Cu-Fe-P cast alloy [J]. Metals and Materials International, 2012, 18(2): 335-339.
[6] KAZUHISA K, KAZUNORI K, RYOICHI M. Dispersions of particles and heat resistance in a Cu-Fe-P alloy [J]. The Society of Materials Science, 2000, 49(5): 482-487. (in Japanese)
[7] CAO H, MIN J Y, WU S D, XIAN A P, SHANG J K. Pinning of grain boundaries by second phase particles in equal-channel angularly pressed Cu-Fe-P alloy [J]. Material Science and Engineering A, 2006, 431: 86-91.
[8] LEI Qian, LI Zhou, WANG Ming-pu, ZHANG Liang, XIAO Zhu, JIA Yan-lin. The evolution of microstructure in Cu-8.0Ni-1.8Si- 0.15Mg alloy during aging [J]. Material Science and Engineering A, 2010, 527: 6728-6733.
[9] XIA Cheng-dong, WANG Ming-pu, ZHANG Wan, JIA Yan-lin, WU Ying-feng, DONG Qi-yi, WEI Hai-gen, XU Gen-ying. Properties and microstructure evolution of hot rolled-quenched Cu-Cr system alloys [J]. The Chinese Journal of Nonferrous Metals, 2012, 22(8): 2230-2237. (in Chinese)
[10] FERNEE H, NAIRN J, ATRENS A. Cold worked Cu-Fe-Cr alloys [J]. Journal of Materials Science, 2001, 36: 5497-5510.
[11] XIA Cheng-dong, WANG Ming-pu, ZHANG Wan, KANG Zhan-yuan, JIA Yan-lin, ZHANG Rui, YU Hong-chun, XU Gen-ying. Microstructure and properties of a hot rolled-quenched Cu-Cr-Zr-Mg-Si alloy [J]. Journal of Materials Engineering and Performance, 2012, 21(8): 1800-1805.
[12] GUO Fu-an, XIANG Chao-jian, YANG Chun-xiu, CAO Xing-min, MU Si-guo, TANG Yu-qiong. Study of rare earth elements on the physical and mechanical properties of a Cu-Fe-P-Cr alloy [J]. Material Science and Engineering B, 2008, 147: 1-6.
[13] DONG Qi-yi, WANG Ming-pu, JIA Yan-lin, XIA Cheng-dong, WANG Wu. Microstructure of as-cast and homogenized Cu-Fe-P-Zn alloys [J]. Journal of Central South University(Science and Technology), 2012, 12(43): 4658-4665. (in Chinese)
[14] EASTERLING K E, WEATHERLY G C. On the nucleation of martensite in iron precipitates [J]. Acta Metallurgica, 1969, 17: 845-852.
[15] ISHIDA I, KIRITANI M. The γ→α transformation mechanism of fine iron precipitates in copper base alloys [J]. Acta Metallurgica, 1988, 36(8): 2129-2139.
[16] HUTCHINSON B. The effect of alloying additions on the recrystallization behavior of copper: A literature review [R]. Swedish Institute of Metals Research Report No. IM-2003, 1985.
[17] XIA Cheng-dong, JIA Yan-lin, ZHANG Wan, ZHANG Ke, DONG QI-yi, XU Gen-ying, WANG Ming-pu. Study of deformation and aging behaviors of a hot rolled-quenched Cu-Cr-Zr-Mg-Si alloy during thermomechanical treatments [J]. Materials and Design, 2012, 39: 404-409.
形变热处理对Cu-2.3Fe-0.03P合金组织与性能的研究
董琦祎1,申镭诺1,汪明朴1,贾延琳1,2,李 周1,曹 峰1,陈 畅2
1. 中南大学 材料科学与工程学院,长沙 410083;
2. 中南大学 冶金与环境学院,长沙 410083
摘 要:应用一种短流程制备技术生产Cu-2.3Fe-0.03P合金带材。合金经过在线热轧淬火以及80%变形量的冷加工后,展示了优良的再结晶抗软化性能。分别利用硬度计及双电桥法测量计算合金的硬度和电导率,利用光学显微镜和透射显微镜观察样品的显微组织。成品Cu-2.3Fe-0.03P合金的主要强化方式为加工硬化,尺寸大约为25 nm的Fe粒子起到稳定冷轧组织的作用。大变形量冷轧会使得合金电导率下降,特别是预时效的样品其电导率下降更多,其主要原因为冷轧过程中小于5 nm的微细粒子容易重新回溶到基体中。实验获得了电导率为66% IACS和硬度为HV 134的Cu-Fe-P合金。
关键词:Cu-Fe-P合金;固溶;热轧;析出;再结晶
(Edited by Mu-lan QIN)
Foundation item: Project supported by Central South University Postdoctoral Science Foundation; Project (CSUZC2013019) supported by the Open Fund for the Precision Instruments of Central South University, China; Project (CSUZC201522) supported by the Open-End Fund for the Valuable and Precision Instruments of Central South University, China
Corresponding author: Yan-lin JIA; Tel: +86-731-88830264; Fax: +86-731-88876692; E-mail: jiayanlin@126.com
DOI: 10.1016/S1003-6326(15)63757-8
Abstract: A short process without solution treatment was developed to manufacture Cu-2.3Fe-0.03P alloy strips. After hot rolling-quenching and cold rolling with 80% reduction, the alloy exhibited excellent resistance to recrystallization softening. The hardness and electrical conductivity of Cu-Fe-P alloy under different thermomechanical treatments were measured by hardness tester and double bridge tester, respectively, and the microstructure of the alloy was examined by optical microscopy and transmission electron microscopy. The results show that the finished product of Cu-2.3Fe-0.03P alloy was strengthened by work hardening, while the Fe precipitates with the size of about 25 nm stabilized the cold rolled structure. The conductivity decreased during cold rolling, especially for the pre-aged specimens, because the fine precipitates with the size smaller than 5 nm re-dissolved easily into the matrix. A Cu-Fe-P alloy with an electrical conductivity of 66% IACS and a hardness of HV 134 can be gained.


