
Fabrication of monolithic bulk Ti3AlC2 and impurity measurement by K-value method
XU Xue-wen(徐学文)1, FU Cheng-ke(富成科)2, LI Yang-xian (李养贤) 1,
ZHU Jiao-qun (朱教群) 3, MEI Bing-chu (梅炳初) 3
1. School of Materials Science and Engineering, Hebei University of Technology, Tianjin 300130, China;
2. Hebei Communications Vocational & Technical College, Shijiazhuang 050091, China;
3. State Key Laboratory of Advanced Technology for Materials Synthesis and Processing, Wuhan University of Technology, Wuhan 430070, China
Received 10 April 2006; accepted 25 April 2006
Abstract:
Polycrystalline bulk Ti3AlC2 material with high purity and density was fabricated by hot pressing from the powder mixture with the starting stoichiometric mole ratios of 2.0TiC/ 1.0Ti/ 1.1Al/ 0.1Si at 1 300-1 500 ℃. X-ray diffraction patterns and scanning electron microscopy photographs of the fully dense samples indicate that the proper addition of silicon is favorable to the formation of Ti3AlC2, consequently results in high purity of the prepared samples. The Ti3AlC2 hot pressed at 1 300 ℃ and 1 400 ℃ is in plane-shape with sizes of 6-8 μm and 15-20 μm in the elongated dimension, respectively. The purities of samples are measured by the K-value method, and the contents of TiC are given by a linear equation.
Key words:
titanium aluminum carbide; hot pressing; K-value method;
1 Introduction
Owing to its distinguished properties, the nanolaminated ternary ceramics Mn+1AXn, or H phase called elsewhere, where M is a transition metal, A is a ⅢA or ⅣA element and X is either carbon and/or nitrogen, n=1, 2, 3, extremely inspired both material and physical scientists with indescribable passions[1-3]. Ti3SiC2 and Ti3AlC2 were typical representatives of H phase. However the former almost abstracted all focuses of researchers because of the convenience of fabrication. Since high purity single-phase Ti3AlC2 was very difficult to obtain, studies on the later were poorly less than those of the former[4-6]. Lots of correlative researches had revealed the unusual properties of Ti3AlC2[7-9]. Ti3AlC2 which was discovered at 1994 has hexagonal crystal structure with space group of D46h-P63/mmc and the crystal lattices, a=0.307 53 nm and c=1.857 8 nm. Similar to the other H phase, Ti3AlC2 possesses a combination of merits of metals and ceramics. Like metals, it is thermally and electrically conductive, easy to machine with ordinary tools, well resistant to thermal shock. Most importantly, the layered ternary that usually has much higher fracture toughness than that of ceramics are outstandingly tolerant to damage, which makes it able to be machined by traditional high-speed steel tools without lubrication or cooling water. Similar to ceramics, Ti3AlC2 is elastically stiff, exhibits excellent high temperature mechanical properties, and has high melting point. Besides the listed properties, the ternary is self-lubricative, and has lower friction coefficient that is even less than those of graphite and MoS2.
Nearly all kinds of fabrication methods, such as HIP, SHS, MA had been applied to synthesize Ti3AlC2. But the phase field of Ti3AlC2 was so narrow that no available processes were obtained nevertheless. Second phases, including TiC, Al2O3 and Ti2AlC, commonly coexisted with Ti3AlC2. In our last studies on the sintering of Ti3SiC2, a novel process was introduced, and the single-phase Ti3SiC2 was sintered by hot-pressing with the additive of aluminum[10]. Consequently, a new process with the additive was suggested to sinter Ti3AlC2 in the work. The K-value method was also used to measure the mass fraction of impurity TiC.
2 ExperimentalThe starting materials used in this work are titanium carbide powders (8.4 μm, 99.2%pure), titanium powders (10.6 μm, 99.0%pure), silicon powders (9.5 μm, 99.5%pure), aluminum powders (12.8 μm, 99.8%pure) and carbon black (13.2 μm, 99%pure). The mixture with a designed composition was mixed in ethanol for 24 h, and then placed in a graphite die with 20 mm in diameter. The samples were sintered in a hot pressing system. The samples were heated in a flowing Ar atmosphere at a rate of 50 ℃/min to the requisite temperature, and held at those temperatures for 2 h under a pressure of 30 MPa. The densities of the sintered products were measured by ARCHIMEDE’s method. And the X-ray diffraction (XRD, Model D/MAX-RB, RIGAKU Corporation, Japan) was used to determine the phases of sintered samples. The microstructures of samples were investigated with scanning electron microscope (SEM, Model JSM-5610LV, JEDL Ltd, Japan).
The K-value method was adopted to ascertain the weight fraction of impurity TiC which was illustrated by XRD patterns. The polished sample was drilled to obtain Ti3AlC2 powders by a high-speed drill. Since the oxidization rate of Ti3AlC2 is much lower than that of TiC, a controlled oxidation at 300 ℃ in air for about 10 h was performed to improve the purity of Ti3AlC2. The products were TiO2, Al2O3, Ti3AlC2 and a little TiC. After dissolving Al2O3 and TiO2 by hydrofluoric acid, Ti3AlC2 powders were completely pure. The mixed powders in which the Ti3AlC2 and TiC powders with the mass ratios from 95∶5 to 20∶80 were uniformly mixed in a carnelian bowl with ethanol. XRD patterns of these mixed powders were carefully measured by the before-mentioned X-ray diffractometer with the step-scan model.
3 Results and discussion3.1 Phase composition of samples sintered by different processes
We had carried out a series of researches on fabrication of Ti3AlC2 with different raw materials, such as Ti/Al/C, TiC/ Al/Ti, TiC/Al/Si/Ti, and sintering processes. Except the case detailed discussed as below, no appropriate routes were available. Fig.1(a) shows XRD patterns of samples sintered by the mixture powders with the stoichiometric mole ratios of 2.0TiC/ 1.0Ti/ (1.2-1.0) Al/ (0-0.2) Si at 1 300 ℃. Except the peaks belonged to main product Ti3AlC2, the weak peaks belonged to TiC were also observed in the patterns which reflected on phase compositions of the samples with starting mixture powders 2.0TiC/ 1.0Ti/ 1.2Al and 2.0TiC/ 1.0Ti/ 1.15Al/ 0.05Si. As the molar ratio of additive silicon increased to 0.1, no phase but Ti3AlC2 was identified by X-ray diffraction, which indicated that the products were completely pure Ti3AlC2. As the cont-

Fig.1 (a) XRD patterns of samples sintered at 1 300 ℃ by different starting mixed powders; (b) XRD patterns of samples sintered by hot pressing mixture powder 2.0TiC/ 1.0Ti/ 1.1Al/ 0.1Si in mole ratio at different temperatures
ent of silicon increased to 0.2, the peaks belonged to the other laminated ternary Ti3SiC2 appearing at 39.6?. The superfluous additive was harmful to sintering of monolithic Ti3AlC2. In conclusion, the appropriate start-
ing powders adopted to sinter high purity Ti3AlC2 were 2.0TiC/ 1.0Ti/ 1.1Al/ 0.1 Si. Generally, a monolithic sample was synthesized with the specific mixed powders when the sintered temperature increased to the range from 1 300 ℃ to 1 500℃, as shown in Fig. 1(b).
Fig.2 shows the scanning electron microscope photographs of the fracture faces of Ti3SiC2 material sintered from 2.0TiC/1.0Ti/1.1Al/0.1Si at 1 300 ℃ and
1 400 ℃. And the morphology characteristics indicated that Ti3AlC2 was in thin plate-shape, with the sizes of 6-8 μm and 15-20 μm in elongated dimension at 1 300 ℃ and 1 400 ℃, respectively. From Fig.2, we can see the samples sintered at these two temperatures were very dense with the density of 4.19 and 4.22 g/cm3 measured by ARCHIMEDE’s method, respectively. With a comprehensive view, the interesting conclusion was pointed out that as an introduced impurity the additive silicon was necessary to the formation of high purity Ti3AlC2.
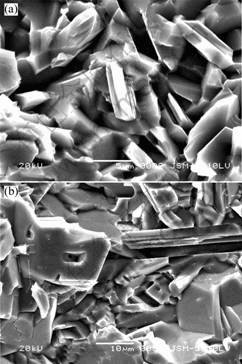
Fig.2 Morphology characteristics of samples with start mole ratio 2.0TiC/1.0 Ti /1.1Al /0.1Si sintered by hot pressing at: (a) 1 300 ℃; (b) 1 400 ℃
3.2 Purity estimation of Ti3AlC2 by K-value method
As a normal quantitative analysis method, the K-value measurement, which is derived from the internal standard method, is based on the followed principle that the intensities of diffraction peaks reflected on different phases in XRD patterns are in direct proportion to their contents. The content of a substance in the mixture with several phases can be measured by XRD patterns with a standard substance s whose content is available. The intensities of peaks, usually the strongest peaks, belonged to j and s substance exhibit the following dependence:
![]() (1)
(1)
where wj and ws are the mass fraction of j substance and s substance, respectively; ρj is the density of j substance; ρs is the density of s substance; Dj and Ds are constants which are correlative to the crystal structure and diffraction absorption of these two substances; Kjs is a coefficient.
When there are only two substances in the mixture, the calibration substance is not necessary. And the mass fractions of these two substances, w1 and w2, yield:
![]() (2)
(2)
As shown in Fig.3, the second phase TiC usually existed in Ti3AlC2 samples sintered by hot pressing with different routes. According to the primary principle referred hereinbefore, the (104) peak located at 2θ=39.06? belonged to Ti3AlC2, and the (111) peak located at 2θ=35.8? belonging to TiC are selected to calculate the content of the second phase. Consequently, Eqn.(2) can be written as
![]() (3)
(3)
where wT and wA are the mass fractions of TiC and Ti3AlC2, IA(104) and IT(111) are intensities of (104) peak and (111) peak belonged to Ti3AlC2 and TiC, respectively.
Fig.4 shows the XRD patterns of several kinds of mixture powders consisted of Ti3AlC2 and TiC. IA(104) and IT(111) were calculated by the approximate integral areas of their peaks. The dependence between IA(104) / IT(111) against wT is summarized in Fig.5 in which a linear law is obviously observed, and can be written as
![]() (4)
(4)
So, the mass fraction of TiC is given by
![]() (5)
(5)
According to Eqn.(5), we measured the mass fractions of

Fig.3 XRD patterns of mixture powders of Ti3AlC2 containing small quantity of TiC
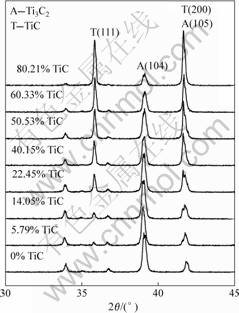
Fig.4 XRD patterns of mixture powders consisting of Ti3AlC2 and TiC obtained by step scan
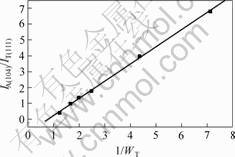
Fig.5 Calibration lines for TiC content in product TiC in samples sintered with 2TiC/Ti/1.1Al/0.1Si, which were 6.67 %, 3.28 % and 3.58 % at 1 300 ℃,1 400 ℃ and 1 500 ℃, respectively.
4 Conclusions
A novel route for the fabrication of high purity Ti3AlC2 was given in this work. The single-phase bulk Ti3AlC2 was synthesized by hot pressing with the mixture powders 2.0TiC /1.0Ti /1.1Al /0.1Si in molar ratio under the pressure of 30 MPa in Ar atmosphere at
1 400 ℃ and 1 500 ℃. The appropriate silicon addition was beneficial to improving the purity of product. The crystal was laminate with the dimensions of 6-8 μm and 15-20 μm synthesized at 1 300 ℃ and 1 400 ℃, respectively. The equation of mass fraction of TiC versus the relative intensities of the peaks belonging to Ti3AlC2 and TiC was also given to calculate the content of impurity.
References[1] BARSOUM M W, EL-RAGHY T. Synthesis and characterization of a remarkable ceramic: Ti3SiC2 [J]. J Am Ceram Soc, 1996, 79(7): 1953-1956.
[2] BARSOUM M W, YAROSCHUCK G, TYAGI S. Fabrication and characterization of M2SnC (M=Ti, Zr, Hf and Nb) [J]. Sc Mater, 1997, 10: 1583-1591.
[3] BARSOUM M W. The MN+1AXN phases: A new class of solids; thermodynamically stable nanolaminates [J]. Prog Solid St Chem, 2000, 28: 201-281.
[4] PIETZKA M, SCHUSTER J C. The ternary boundary phase of the quaternary system Ti-Al-C-N[A]. In Leuven Proceedings, Part A: Commision of the European Communities[C], Brussels, Belgium, 1992.
[5] PIETZKA M, SCHUSTER J C. Summary of the constitutional data on the Al-Ti-C system [J]. J Phase Equilibrium, 1994, 15 (4): 392
[6] GE Z B, CHEN K X, GOU J M, et al. Combustion synthesis of ternary carbide in the Ti-Al-C system [J]. J Euro Ceram Soc, 2003, 23: 567-574
[7] .TZENOV N V, BARSOUM M W. Synthesis and Charicterization of Ti3AlC2 [J]. J Am Ceram Soc, 2000, 83(4): 825-832.
[8] CHEN K X, GUO J M, GE Z B, et al. A novel route for preparing Ti3AlC2 ceramics [J]. Rare Metal Mater Eng, 2002, 31(3): 20-23.
[9] WANG X H, ZHOU Y C. Solid-liquid reaction synthesis of layered machinable Ti3AlC2 ceramic [J]. J Mater Chem, 2002, 12(3): 455-460.
[10] ZHU J Q, MEI B C, XU X W, et al. Effect of aluminum on the reaction synthesis of ternary carbide Ti3SiC2 [J]. Scr Mater, 2003, 49: 693-697.
Corresponding author: LI Yang-xian; Tel: +86-22-26582214; E-mail: nextnest@eyou.com


