
Ultrasonic-electrodeposited Sn-CNTs composite used as anode material for lithium ion battery
LI Chang-ming(李昌明)1, 2, 3, ZHANG Ren-yuan(张仁元)2, LI Wei-shan(李伟善)1
ZHAO Ling-zhi(赵灵智)1, 2, HU She-jun(胡社军)1, 3, RAO Mu-min(饶睦敏)1, XU Jian-xia(徐建霞)2
1. School of Chemistry and Environment, South China Normal University, Guangzhou 510006, China;
2. Faculty of Material and Energy, Guangdong University of Technology, Guangzhou 510090, China;
3. Department of Mathematics and Physics, Wuyi University, Jiangmen 529020, China
Received 15 July 2007; accepted 10 September 2007
Abstract:
Tin carbon nanotube (Sn-CNTs) composite was prepared by ultrasonic-electrodepositing tin on the substrate of copper foil in a sulfate bath containing carbon nanotubes (CNTs). The composites were characterized by scanning electron microscopy (SEM), cyclic voltammetry(CV) and charge-discharge cycling test. The results show that Sn-CNTs have a better electrochemical performance than Sn. The capacity of Sn-CNTs is 843 mA?h/g during the first discharge and the efficiency of charge-discharge reaches 85%. After 50 cycles, the capacity of Sn-CNTs keeps at 380 mA?h/g. The CNTs in tin act as a structure supporter and play a role of an elastomer and conductive network, alleviating the electrode dilapidation resulted from volume change during the lithium insertion and deinsertion.
Key words:
lithium ion battery; anode materials; electrodeposition; tin; carbon nanotube;
1 Introduction
Carbonaceous materials are currently used as anode in commercial lithium ion batteries. With the development of electric vehicles (EV) for reducing environment pollution and portable electronic devices, there is an interest in developing new electrode materials with higher capacity, higher stability, higher safety, and lower cost for lithium ion batteries.
Many new high capacity materials have been studied recently[1]. Tin-based anodes are attractive anode materials due to its high-capacity. They have been considered alternative options for many years. Tin anodes have been reviewed and investigated in detail[2]. However, the main drawback of this material is the drastic volume change due to lithium insertion and deinsertion to host materials during charge/discharge cycles[3-5]. The volume change often results in huge mechanical stress, which leads to poor electrochemical performance[6].
In this work, an idea of constructing web in active materials was introduced and it was realized by preparing tin carbon nanotubes (Sn-CNTs) with ultrasonic-electrodeposition process. The parameters for the electrodeposition were obtained through orthogonal experiments. The morphology and the electrochemical performance of the prepared Sn-CNTs were studied by SEM, CV and charge-discharge test.
2 Experimental2.1 Preparation of tin carbon nanotube composite
The Sn-CNTs composite was prepared by ultrasonic-electrodepositing tin on copper foil (thickness of 10 ?m) in a sulfate bath, under ultrasonic conditions with a constant current density of 0.375 A/dm2 at 35 ℃, and using a tin plate(purity of 99.9%) as counter electrode, the waves was acted when depositing. The sulfate bath contains SnSO4(30 g/L), sulfuric acid(100 mL/L), and additive SS-820, SS-821, and the carbon nanotubes(3 g/L). Tin film was prepared under the same conditions with no CNT in bath. These films were cleaned and used as working electrode for the performance determination.
2.2 Performance determination
As comparison a CNTs electrode was fabricated by filling slurries of 90%(mass fraction) CNTs and 10% polyvinylidene fluoride (PVDF) dissolved in N-methylpyrrolidinone(NMP) onto copper foil. The electrodes were dried at 60 ℃ in vacuum before using.
The working electrode was tested in an electrochemical cell using lithium sheet as both counter and reference electrodes. The electrolyte was 1 mol/L LiPF6 in an organic liquid mixture consisting of ethylene carbonate (EC), ethyl methyl carbonate (EMC) and diethyl carbonate (DEC) (1?1?1 in volume ratio). A simple two-electrode cell was fabricated in a dry argon-filled glove box. Cyclic voltammetry(CV) was conducted at 1 mV/s between open-circuit potential and 0 V using a potentiostat (Solartron 1480). Charge and discharge tests were carried out at a constant current of 56 ?A/cm2, i.e., 1/5C rate, between 1.5 and 0 V (vs Li/Li+) using the same potentiostat. SEM images were obtained on a SEM system (JSM-6380LA). All measurements were carried out at room temperature.
3 Results and discussion3.1 Morphology of Sn-CNTs
Fig.1 shows the SEM image of Sn-CNTs electrode. It can be seen that the CNTs were inserted into tin particles, and uniformly distributed. CNTs can provide a path for lithium ion insertion. Active materials can be used efficiently because the diffusion of lithium ions becomes easier. Moreover, the CNTs may consolidate the tin body, reduce the breaking up of electrode, act as bridges for electrons and reduce the volume change.

Fig.1 SEM image of ultrasonic-electrodeposited Sn-CNT composite electrode
3.2 Electrochemical performances of Sn-CNTs
Fig.2 shows the cyclic voltammograms of the Sn-CNT composite electrode at 1 mV/s. As can be seen from Fig.2 that during the first cathodic potential sweeping, a reduction peak appears at 1.35 V, which is associated with catalytic decomposition of electrolyte and solid electrolyte interphase (SEI) is formed according to the previous studies[7-8]. As the potential becomes more negative, another peak appeared at 0.6 V, which is attributed to the formation of lithium-poor alloying phase, as the potential becomes more negative, a peak appears at 0.25 V, lithium-rich alloying phase is formed[9-10].
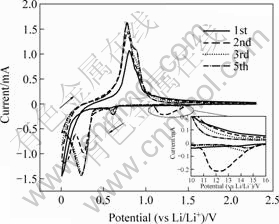
Fig.2 Voltammograms of Sn-CNT composite electrode (Scan rate: 1 mV/s)
During the second sweeping, the peak at 1.35 V is higher than that of the first cycle. This indicates that the SEI film formed on electrode at the first cycle is not enough, because the electrode cracks during the first cycle, and the surface area is enlarged.
Different behaviors of Sn-CNTs, Sn and CNTs were observed, the results are shown in Fig.3. During the first cathodic sweeping, the current of CNTs electrode is observed at 1.1 V. As the potential becomes more negative, the current increases, and a peak appears at 0.28 V, which is assigned to SEI film formed on CNTs electrode at the first cycle, but the Sn-CNTs electrodes have no such current. From Fig.3 it can be seen that Sn and the Sn-CNTs curvesare very similar, the current for lithium insertion and deinsertion on Sn-CNTs electrode is larger than that on Sn, indicating that the insertion/deinsertion of lithium in Sn is improved by CNTs.
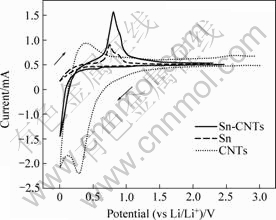
Fig.3 Voltammograms of Sn-CNTs, Sn and CNTs for first cycle (Scan rate: 1 mV/s)
Fig.4 shows the first charge and discharge curves of different electrodes. The discharge capacity of Sn-CNTs electrode is 843 mA?h/g, larger than those of Sn and CNTs. The irreversible capacity of Sn-CNTs 161 mA?h/g, smaller than Sn, which is 120 mA?h/g. In addition, the discharge plateau of Sn-CNTs electrode is higher than Sn, indicating that the CNTs make the Li ion insertion/deinsertion in tin easier.
Fig.5 shows the cyclic performance of the Sn-CNTs electrode. It can be found that the initial capacity is 843mA?h/g. After 50 cycles, the reversible capacity of Sn-CNTs electrodes still maintains 380 mA?h/g.
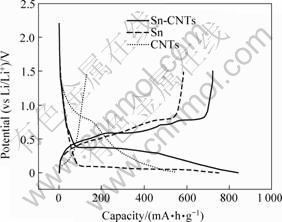
Fig.4 First charge—discharge curves of CNTs, Sn and Sn-CNTs

Fig.5 Cycle performance of Sn-CNTs
4 Conclusions
1) Sn-CNTs can be prepared by codeposition electrochemically under with ultrasonic condition.
2) Sn-CNTs have a better electrochemical performance than tin. The capacity is 843 mA?h/g during the first discharge and the efficiency of charge-discharge reaches 85%. After 50 cycles, the capacity of Sn-CNTs keeps higher than 380 mA?h/g.
References
[1] WINTER M, JURGEN O. Besenhard, electrochemical lithiation of tin and tin-based intermetallics and composites[J]. Electrochim Acta, 1999, 45: 31-50.
[2] TARASCON J M, ARMAND M. Issues and challenges facing rechargeable lithium batteries[J]. Nature, 2001, 414: 359-367.
[3] COURTNEY I A, DAHN J R. Electrochemical and in-situ X-ray diffraction studies of the reaction of lithium with tin oxide composite[J]. J Electrochem Soc, 1997, 144: 2045.
[4] TAMURA N, OHSHITA R, FUJIMOTO M, KAMINO M, FUJITANI S. Advanced structures in electrodeposited tin base negative electrodes for lithium secondary batteries[J]. J Electrochem Soc, 2003, 150(6): A679-A683.
[5] YANG S, ZAVALIJ P Y, WHITTINGHAM M S. Anodes for lithium batteries: tin revisited[J]. Electrochemistry Communications, 2003, 5: 587-590.
[6] WACHTLER M, BESENHARD J O, WINTER M. Tin and tin-based intermetallics as new anode materials for lithium-ion cells[J]. J Power Sources, 2001, 94: 189-193.
[7] LI Chang-ming, HUANG Qi-ming, ZHANG Ren-yuan, LI Wei-shan, ZHAO Ling-zhi, HU She-jun. A comparison of the performances of two kinds of Sn films as lithium-ion insertion electrodes prepared by electrodeposition[J]. Acta Metallurgica Sinica, 2007, 43(5): 515-520.
[8] ZUO X X, XU M Q, LI W S, SU D G, LIU J S. Electrochemical reduction of 1,3-propane sultone on graphite electrode and its application in Li-ion battery[J]. Electrochemical and Solid-State Letters, 2006, 9: A196-A199.
[9] HUGGINS R A. Lithium alloy negative electrodes[J]. J Power Sources, 1999, 81/82: 13-19.
[10] INABA M, UNO T, TASAKA A. Irreversible capacity of electrodeposited Sn thin film anode[J]. J Power Sources, 2005, 146: 473-477.
Foundation item: Projects(2006A10704003,2006Z3-D2031) supported by the Key Projects of Guangdong Province and Guangzhou City, China
Corresponding author: LI Wei-shan; Tel: +86-20-39310256; E-mail: liwsh@scnu.edu.cn


