Trans. Nonferrous Met. Soc. China 24(2014) 3872-3878
Effect of Zr addition on microstructure and properties of Al-Mn-Si-Zn-based alloy
Jun-chao ZHANG1, Dong-yan DING1, Wen-long ZHANG1, Shao-hai KANG1, Xing-long XU1, Yong-jin GAO2, Guo-zhen CHEN2, Wei-gao CHEN2, Xiao-hua YOU2
1. School of Materials Science and Engineering, Shanghai Jiao Tong University, Shanghai 200240, China;
2. Huafon Nikki Al Co., Ltd., Shanghai 201506, China
Received 17 October 2013; accepted 5 November 2014
Abstract:
Effects of Zr addition on the microstructure, mechanical and electrochemical properties of Al-Mn-Si-Zn alloy were investigated. Transmission electron microscopy (TEM) observations reveal that, in as-annealled state, the precipitates in the Zr-containing alloy are finer and more dispersive than those in the Zr-free alloy. Whereas, in simulated brazing state, a weaker precipitation is found in the Zr-containing alloy. Tensile testing results indicate that, with Zr additon, comprehensive mechanical properties of the as-annealed alloys could be significantly improved but weakened for the simulated brazing alloy. Electrochemical testing results reveal that, with Zr addition, the corrosion resistance of the as-annealed alloy decreases. However, after the simulated brazing treatment, such a negative effect of Zr element on the corrosion behavior of the alloy could be negligible.
Key words:
aluminium alloy; Zr alloying; precipitation; corrosion; mechanical property;
1 Introduction
Zr is one of the important alloying elements to improve the microstructures and properties of aluminum alloys. When Zr is added to aluminum alloys, Zr element may exist in four kinds of forms at different heat processing and heat-treatment stages, i.e., solid solution in matrix, coarse primary Al3Zr phase, metastable Al3Zr phase as well as equilibrium Al3Zr phase. If the amount of Zr addition is too high, coarse primary Al3Zr dendritic phases will form and aggravate the mechanical properties of the Al alloys [1]. The metastable Al3Zr phase is usually much finer and coherent with the matrix with a mismatch ratio of only 0.8%. It is a kind of very effective strengthening dispersoid, which can inhibit recrystallization, refine the microstructure and increase the recrystallization temperature and strength of the matrix alloys [2-7]. Whereas, the equilibrium Al3Zr phase is usually coarse and incoherent with the matrix [8-12].
It was reported that metastable Al3Zr phase in Al-0.5% Zr alloy could transform to equilibrium Al3Zr phase after several hours of annealing at 640 °C. At a lower temperature like 500 °C, such a phase transformation will take a longer annealing time more than 120 h [13]. For Al-0.18%Zr alloys metastable Al3Zr phase could not transform to equilibrium Al3Zr phase even after 700 h of annealing at 460 °C. However, equilibrium Al3Zr phase could directly form from the solid solution cooled down at certain situations (higher temperature) [14]. Large quantity of equilibrium η-Al3Zr phase particles could form in the Al-0.5%Zr alloy which was slowly cooled down from the annealling temperature of 600 °C [15,16]. The migration of large angle grain boundaries often resulted in the change of crystallography relationship between Al3Zr particles and the matrix from coherent or half-coherent to incoherent state [2].
Zr addition to Al-Zn-Mg or Al-Cu alloys could result in inhibited recrystallization, increased recrystallization temperature, improved strength and fracture property as well as anti-corrosion behavior of the alloys. SUN et al [17] found that Zr addition could lead to the formation of homogeneously distributed skeleton-like non-eutectic phase (Al2CuMg+AlZnMgCu) and secondary precipitates. LI et al [16] found that Zr element preferred to refine the as-cast and recrystallized microstructures. And the strength, hardness and elongation of the alloys increased with increase of the Zr content. When the Zr content was over 0.19%, coarse Al3Zr phase formed in the as-cast microstructure and the mechanical properties of the alloys were aggravated. And for 2159Al alloy, the appropriate Zr content was 0.19% [1]. An intergranular corrosion which is near the grain boundaries is a relatively common corrosion form [18]. If the alloy happens in this form of corrosion, the alloy strength may drop dramatically, which often causes a catastrophic consequence. ZHANG et al [19] reported that the anti-intergranular corrosion property of 7055Al alloys could be improved with increase of the Zr content from 0 to 0.15% .
To date, few literatures have been reported on the effect of Zr addition on Al-Mn-based alloys although Zr element has been used to enhance the brazing properties of modified 3003Al alloys. Therefore, in the present work, the effect of Zr content on the microstructure and properties of an Al-Mn-Si-Fe-Zn alloy was studied, which has been developed for heat-exchange applications. This comprehensive investigation should help for a better understanding of the role of Zr element in affecting the annealing and heat-treatment microstructures, mechanical properties and corrosion behaviors.
2 Experimental
The chemical composition of the aluminum alloys is shown in Table 1. All of the alloys contained the same contents of Mn, Si, Fe and Zn elements but different content of Zr element. Corresponding alloy foils with a thickness of 0.1 mm were produced through the same processes, i.e., casting and hot rolling as well as cold rolling. After a finish rolling, some alloy foils were annealed at 400 °C for 2 h. And other alloy foils were heat-treated at 600 °C for 5 min in a furnace and then quickly cooled down to room temperature, which is a simulation of the brazing process.
Table 1 Chemical composition of alloys (mass fraction, %)

Microstructures of the heat-treated alloys were characterized with JEM-2100F transmission electron microscope (TEM). The TEM specimens were prepared through electrolytic polishing in 4% HClO4 and methanol solutions, followed by an ion beam thinning. Tensile testings were carried out on Zwick universal tensile machine. The tensile rate was 1 mm/min. Five tensile samples were used for obtaining the average values of mechanical properties. The tensile fracture surfaces of the alloys were examined with a field emmision scanning electron microscope (SEM, FEI SIRION 200).
Specimens for the electrochemical experiments were degreased with acetone and rinsed in distilled water. Three parrallel specimens for each testing were used to confirm the experimental results. The electrochemical testing with 0.5% NaCl solution was carried out at room temperature. Tafel measurements were performed in a three-electrode system with a CHI660C interface. The specimen, saturated calomel electrode (SCE) and a platinum plate were used as the working electrode, reference electrode and counter electrode, respectively. The potential sweep rate was 1 mV/s. Before testing, the alloy sample was stabilized for 30 min in the electrolyte. All potentials were reported versus those of the SCE. The corrosion surfaces obtained after the electrochemical testings were also examined by SEM.
3 Results and discussion
3.1 Microstructure
Figure 1 shows TEM images of the precipitates in the as-annealed alloys. Although some micrometer-sized precipitates may be found in the alloys, the size of most of the precipitates in the Zr-free alloy usually varies from several tens of nanometers to 200 nm. These precipitates are found to be AlMnSi and AlMnFeSi phases according to the EDX analyses. And most of them have an ellipsoidal shape (see Fig. 1(a)). Compared with the Zr-free alloy, the Zr-containing alloy has a finer and denser precipitation (see Fig. 1(b)). It is obvious that the Zr addition to the alloy could refine and promote the precipitation of the alloy, resulting in uniformly distributed precipitates. However, the Zr-additon has no much effect on the morphology of the precipitates in the as-annealed alloy.
Figure 2 shows TEM images of the simulated brazing alloys. In comparison with the precipitates in the as-annealed alloys, the precipitates in the simulated brazing alloys present quite different precipitation densities and slight in different shapes. Although the size of the precipitates is usually ranging from several tens of nanometers to 200 nm, the Zr-containing alloy has a lower precipation density than the Zr-free alloy, which is quite different from the as-annealed alloys. This suggests that for the simulated brazing alloys, Zr addition mainly affects the density of the precipates in the alloys, but has little effect on the size and morphology of the precipates.

Fig. 1 TEM micrographs of as-annealed alloys

Fig. 2 TEM micrographs of simulated brazing alloys
During the simulated brazing process (heating up to 600 °C), a large quntity of precipitates in the alloys will dissolve into a(Al) matrix since solid solubility of alloying elements in the simulated brazing alloy matrix is higher than that in the as-annealed alloy matrix at a lower temperature (400 °C). And during the subsequent air-cooling of the simulated brazing process, the cooling rate is relatively higher. Therefore, alloying elements may still super-saturate in the a(Al) matrix. Our experimental results indicate that the Zr addition could restrain the precipitation in the brazing process probably through promoting the dissolution of other alloying elements in the a(Al) matrix.
3.2 Mechanical properties
The tensile testing results of the two kinds of alloys are shown in Table 2. The yield strength, tensile strength and elongation of the as-annealed Zr-free alloy are 61.14 MPa, 127.14 MPa and 13.47%, respectively. Corresponding values of the as-annealed Zr-containing alloy are 75.82 MPa, 138.87 MPa and 15.92%, respectively. It is remarkable that, with 0.2% Zr addition, the yield strength, tensile strength and elongation of the as-annealed alloy have increase of 24.01%, 9.23% and 18.12%, respectively. These facts reveal that the Zr addition could lead to excellent comprehensive mechanical properties for the as-annealed alloys.
Table 2 Tensile testing results of alloys

Table 2 also shows the mechanicla properties of the simulated brazing alloys. The yield strength, tensile strength and elongation of the Zr-free alloy are 56.63 MPa, 145.87 MPa and 11.44%, respectively. Compared with the Zr-free alloy, the yield strength, tensile strength and elongation of the Zr-containing alloy are slightly low. Obviously, mechanical enhancement through the 0.2% Zr addition does not occur for the simulated brazing alloys, which is quite different from the annealed alloys.
It is well known that several strengthening mechanisms such as solution strenthening, grain refining strenthening and precipitate strenthening may decide the mechanical performance of alumnium alloys [20]. Considering the TEM observations that Zr addition could lead to a stronger precipitation in the as-annealed state but a weakened precipitation in the brazing state, we believe that the above mechanical difference in the two kinds of alloys should be mainly caused by the difference in precipitate strengthening. As an important alloying element, Zr may contribute to the precipitation of ultrafine Al3Zr particles, which could be nucleation centers for primary or secondary precipitates. The experimental results on the annealed alloys reveal that finer and denser precipitates could form in the Zr-containing alloy rather than in the Zr-free alloy. Such a uniform and dense precipitation should be favorable for a precipitate strengthening and results in the enhanced mechanical properties. For the simulated brazing alloys the precipitation has been weakened after Zr addition, which is unfavorable for the precipitate strengthening. As a result, the strengthening effect through Zr addition does not appear in the high-temperature heat-treated alloys.
SEM images of the tensile fracture surfaces of both as-annealed and simulated brazing alloys are shown in Figs. 3 and 4. All of the alloys show a ductile fracture with a large quantity of dimples on the fracture surface. Different dimple size and fracture at large precipitates could be observed. The relatively fine dimples on the fracture surfaces of the annealed Zr-containing alloy rather than the annealed Zr-free alloy seem to be a good explaination for the enhanced mechanical property (elongation) through Zr addition.
3.3 Electrochemical properties
Figure 5 presents the Tafel polarization curves of the studied alloys tested in 0.5% NaCl solution. Corresponding electrochemical parameters are listed in Table 3. As shown in Fig. 5(a), the anode branch of the Tafel polarization curve of the Zr-free alloy presents an active dissolution feature with its pitting potential (φpit) highly close to the corrosion potential (φcorr), which suggests that the Zr-free alloy should be sensitive to pitting corrosion. Compared with the Zr-free alloy, the φcorr of the Zr-containing alloy is -877 mV, which is much lower than that (-763 mV) of the Zr-free alloy (see Table 3). With Zr addition to the alloy, both the cathode reaction and the anode reaction are enhanced. And the polarization curve moves to the high current density area, which indicates that the corrosion reaction of Al alloy is enhanced. The corrosion current density of the Zr-free alloy is 34.05 μA/cm2, while the corrosion current density of the Zr-containing alloy is 124.3 μA/cm2 (see Table 3). This indicates that 0.2% Zr addition to the alloy will aggravate the corrosion resistance of the annealed alloy in the NaCl solution.

Fig. 3 SEM images showing tensile fracture of as-annealed alloys
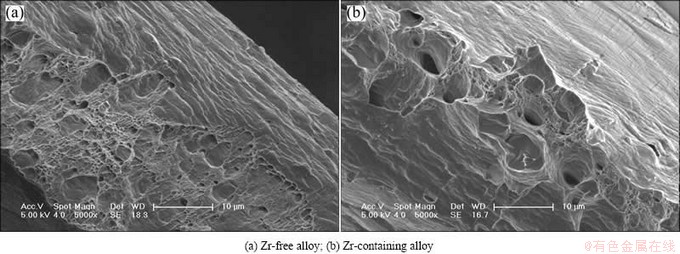
Fig. 4 SEM images showing tensile fracture of simulated brazing alloys

Fig. 5 Tafel polarization curves of alloys
Table 3 Tafel polarization parameters of alloys tested in 0.5% NaCl solution
Figure 6 shows the corrosion surface of the annealed alloys tested in the NaCl solution. Many corrosion pits are visible on the surface of the Zr-free alloy (see Fig. 6(a)), which shows a pitting feature. Whereas, the corrosion characteristics of the Zr-containing alloy belong to an evident intergranular corrosion. Obviously, 0.2% Zr addition to the alloy results in a great change of the corrosion behavior of the annealed alloy.
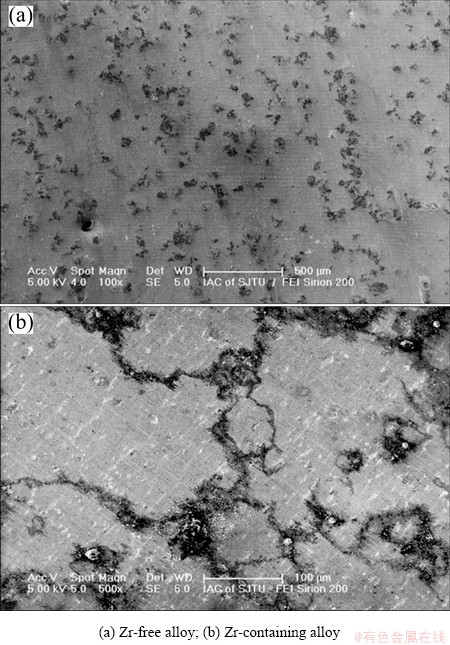
Fig. 6 Corrosion surface of annealed alloys tested in 0.5% NaCl solution
Figure 5(b) also shows the Tafel polarization curves of the simulated brazing alloys tested in 0.5% NaCl solution. Polarization curves of the two kinds of alloys nearly coincide with each other. As shown in Table 3, the corrosion potentials of the Zr-free alloy and Zr-containing alloy are -718 mV and -715 mV, respectively. Unlike the intergranular corrosion for the annealed alloy, the simulated brazing Zr-containing alloy demonstrates a pitting corrosion (see Fig. 7), which is similar to that of the Zr-free alloy. This indicates that the adverse effect of Zr addition on the corrosion performance of the alloy could be greatly minimized after the brazing treatment.
According to the dilution theory for explanation of the intergranular corrosion mechanism of aluminium alloys, the difference of corrosion potential between grain boundary anode phase or precipitate free zone (PFZ) and matrix contributes to the galvanic corrosion and then evolutes intergranular corrosion. This theory considers that corrosion cells can form among grain boundary precipitates, PFZ and inter-grains when the Zr-free alloys are put in corrosion media. The grain boundary precipitates may be anode or cathode phase. While the PFZ tends to become anode and dissolves at the first stage, which provides rapid propagation path for the intergranular corrosion. Therefore, an intergranular corrosion is closely related to the microstructural characteristics such as the grain structure, grain boundary precipitates and PFZ of the alloys. In the present study, the corrosion is greatly affected by the Zr content of the alloy and the heat-treatment process.

Fig. 7 Corrosion surface of simulated brazing alloys tested in 0.5% NaCl solution
The maximum dissolution degree of Zr element in Al alloys was reported as 0.11% [21]. For Zr-containing alloy, the Zr content is 0.2%. Therefore, Al3Zr phases will inevitably form in the as-cast and as-annealed alloys. Ultrafine Al3Zr phases or Zr-induced precipitates may precipitate along the grain boundaries during the annealing process. And Zr-free zone (PFZ) will form around the Zr-related precipitates at the grain boundary areas. The Zr-related precipitates act as cathode phases due to their potential higher than that of the matrix, and the PFZ acts as anode phase which has a lower potential than the matrix. In the NaCl solution, the corrosion cells which formed among the Zr-related precipitates and the PFZ as well as the matrix should have contributed to the formation of fast corrosion channels and propagated cracks of the intergranular corrosion. High temperature brazing treatment will dissovle alloying elements or precipitates into the Al matrix and thus minimize microstructural difference of the alloys. Therefore, the above mentioned factors favorable for an intergranular corrosion should have been minimized through the simulated brazing treatment. Thus, it is not strange that the corrosion type of the simulated brazing Zr-containing alloy could not demonstrate an intergranular corrosion.
4 Conclusions
1) 0.2% Zr addition to Al-Mn-Si-Zn alloys could result in the formation of finer and denser precipitates in the as-annealed alloy rather than in the simulated brazing alloy. In as-annealed state, the yield strength, tensile strength and elongation of the Al-Mn-Si-Zn alloy can be enhanced by the Zr addition. But in the simulated brazing state, such an mechanical enhancement does not occur.
2) The corrosion potential of the Al-Mn-Si-Zn alloy is greatly affected by the Zr alloying. The 0.2% Zr addition makes the corrosion potential shift to negative direction and changes the corrosion type of the as-annealed alloy from pitting corrosion to intergranular corrosion. However, the negative effect of 0.2% Zr addition on the corrosion behavior of the alloy can be minimized through the simulated brazing treatment.
Acknowledgment
The authors would like to thank the Instrumental Analysis Center of Shanghai Jiao Tong University for SEM experiments and Prof. Bin CHEN for TEM experiments.
References
[1] HIDEO Y, YOSHIO B. The role of zirconium to improve strength and stress-corrosion resistance of Al-Zn-Mg and A-Zn-Mg-Cu alloys [J]. Transactions of the Japan Institute of Metals, 1982, 23: 620-630.
[2] GUO Jun-qing, OHTERA K. An intermediate phase appearing in Ll2-Al3Zr to DO23-Al3Zr phase transformation of rapidly solidified Al-Zr alloys [J]. Materials Letters, 1996, 27: 343-347.
[3] GANDEL D S, EASTON M A, GIBSON M A, BIRBILS N. Influence of Mn and Zr on the corrosion of Al-free Mg alloys, Part 2: Impact of Mn and Zr on Mg alloy electrochemistry and corrosion [J]. Corrosion Science Section, 2013, 8: 744-751.
[4] VLACHA M, STULIKOVA I, SMOLA B, PIESOVA J, CISAROVA H, DANISA S, PLASEK J, GEMMA R, TANPRAYOON D, NEUBERT V. Effect of cold rolling on precipitation processes in Al-Mn-Sc-Zr alloy [J]. Materials Science and Engineering A, 2012, 548: 27-32.
[5] LI Bo, PAN Qing-lin, HUANG Xing, YIN Zhi-min. Microstructures and properties of Al-Zn-Mg-Mn alloy with trace amounts of Sc and Zr [J]. Materials Science and Engineering A, 2014, 616: 219-228.
[6] VANDALEN M E, GYGER T, DUNAND D C, SEIDMAN D N. Effects of Yb and Zr microalloying additions on the microstructure and mechanical properties of dilute Al-Sc alloys [J]. Acta Materialia, 2011, 59: 7615-7626.
[7] POKOVA M, CIESLAR M, LACAZE J. TEM investigation of precipitation in Al-Mn alloys with addition of Zr [J]. Manufacturing Technology, 2012, 13(12): 212-217.
[8] KNIPLING K E, KARNESKY R A, LE C P, DUNAND D C, SEIDMAN D N. Effects of minor Zr and Er on microstructure and mechanical properties of pure aluminum [J]. Materials Science and Engineering A, 2010, 58: 5184-5195.
[9] BOOTH-MORRISON C, DUNAND D C, SEIDMAN D N. Coarsening resistance at 400 °C of precipitation-strengthened Al-Zr-Sc-Er alloys [J]. Acta Materialia, 2011, 59: 7029-7042.
[10] JIA Zhi-hong, ROYSET J, SOLBERG J K, LIU Qing. Formation of precipitates and recrystallization resistance in Al-Sc-Zr alloys [J]. Transactions of Nonferrous Metals Society of China, 2012, 22(8): 1866-1871.
[11] WANG Ying, PAN Qing-lin, SONG Yan-fang, LI Chen, LI Zhi-feng, CHEN Qin, YIN Zhi-min. Recrystallization of Al-5.8Mg-Mn-Sc- Zr alloy [J]. Transactions of Nonferrous Metals Society of China, 2013, 23: 3235-3241.
[12] FANG Hua-chan, CHAO Hong,CHEN Kang-hua. Effect of Zr, Er and Cr additions on microstructures and properties of Al–Zn–Mg–Cu alloys [J]. Materials Science and Engineering A, 2014, 610: 10-16.
[13] NES E. The effect of a fine particle dispersion on heterogeneous recrystallization [J]. Acta Metallurgica, 1976, 24: 391-398.
[14] DESCHAMPS A, BRECHET C. Nature and distribution of quench-induced precipitation in an Al-Zn-Mg-Cu alloy [J]. Scripta Materialia, 1998, 39: 1517-1522.
[15] SENATOROVA O G, UKSUSNIKOV A N, LEGOSHINA S F, FRIDLYANDER I N, ZHEGINA I P. Influence of different minor additions on structure and properties of high-strength Al-Zn-Mg-Cu alloy sheets [J]. Forum of Materials Science, 2000, 31: 1249-1253.
[16] LI Hui-zhong, ZHANG Xin-ming, CHEN Ming-an, GONG Min-ru, ZHOU Zhuo-ping. Effect of Zr content on microstructures and properties of 2519 aluminum alloy [J]. Heat Treatment of Metals, 2009, 24: 11-16. (in Chinese)
[17] SUN Li-ming, YU Hua-shun, MIN Guang-hui, ZHANG Jiang. The effect of Zr on the microstructure and property of Al-Zn-Mg-Cu alloy [J]. Special Casting and Nonferrous Alloys, 2007, 27(5): 380-381. (in Chinese)
[18] ZHANG Wei-long, FRANKEL G S. Transitions between pitting and intergranular corrosion in AA2024 [J]. Electrochimica Acta, 2003, 48: 1193-1210.
[19] ZHANG Xin-ming, LIU Sheng-dan, LIU Ying, ZHANG Xiao-yan. Influence of quench rate and zirconium content on intergranular corrosion of 7055 type aluminum alloy [J]. Science and Technology, 2007, 38: 181-186. (in Chinese)
[20] LI Hai, ZHENG Zi-qiao, WANG Zhi-xiu. Investigation of secondary ageing characteristics of 7055 aluminum alloy (II): Microstructures and fractography [J]. Rare Metal Materials and Engineering, 2005, 34: 1230-1235. (in Chinese)
[21] XIE You-hua, YANG Shou-jie, DAI Sheng-long. Application of element Zr in aluminum alloys [J]. Journal of Aeronautical Materials, 2002, 16(7): 14-17. (in Chinese).
Zr元素对Al-Mn-Si-Zn系铝合金组织与性能的影响
张俊超1,丁冬雁1,张文龙1,康绍海1,徐兴隆1,高勇进2,陈国桢2,陈为高2,尤小华2
1. 上海交通大学 材料科学与工程学院,上海 200240;
2. 华峰日轻铝业股份有限公司,上海 201506
摘 要:研究Zr添加对Al-Mn-Si-Zn合金显微组织、力学性能及电化学性能的影响。透射电镜观察表明,Zr合金化可使退火态合金中的析出相更加细小、弥散,但是对模拟钎焊态合金的影响较弱。拉伸实验结果表明,添加Zr能够显著提高退火态合金的力学性能,但是对模拟钎焊态合金影响不大。电化学实验结果则表明,Zr元素的添加可降低退火态合金的抗腐蚀性能,但是对模拟钎焊态合金的影响不大。
关键词:铝合金;Zr合金化;析出相;腐蚀;力学性能
(Edited by Xiang-qun LI)
Foundation item: Proejct (51171108) supported by the National Natural Science Foundation of China; Project supported by Shanghai High-tech Development Project, China
Corresponding author: Dong-yan DING; Tel: +86-21-34202741; E-mail: dyding@sjtu.edu.cn
DOI: 10.1016/S1003-6326(14)63545-7
Abstract: Effects of Zr addition on the microstructure, mechanical and electrochemical properties of Al-Mn-Si-Zn alloy were investigated. Transmission electron microscopy (TEM) observations reveal that, in as-annealled state, the precipitates in the Zr-containing alloy are finer and more dispersive than those in the Zr-free alloy. Whereas, in simulated brazing state, a weaker precipitation is found in the Zr-containing alloy. Tensile testing results indicate that, with Zr additon, comprehensive mechanical properties of the as-annealed alloys could be significantly improved but weakened for the simulated brazing alloy. Electrochemical testing results reveal that, with Zr addition, the corrosion resistance of the as-annealed alloy decreases. However, after the simulated brazing treatment, such a negative effect of Zr element on the corrosion behavior of the alloy could be negligible.


