
A novel recovery technology of trace precious metals from waste water by combining agglomeration and adsorption
ZOU Hua-sheng(邹华生), CHU Zhu-qiang(储著强), LIN Gang(林 岗)
School of Chemical and Energy Engineering, South China University of Technology, Guangzhou 510640, China
Received 30 September 2006; accepted 20 April 2007
Abstract:
A novel and efficient technology for separating and recovering precious metals from waste water containing traces of Pd and Ag was studied by the combination of agglomeration and adsorption. The recovery process and the impacts of operating conditions such as pH value of waste water, adsorption time, additive quantity of the flocculant and adsorbent on the recovery efficiency were studied experimentally. The results show that Freundlich isothermal equation is suitable for describing the behavior of the recovery process, and the apparent first-order adsorption rate constant k at 25 ℃ is about 0.233 4 h-1. The optimum technology conditions during the recovery process are that pH value is 8-9; the volume ratio of flocculant to waste water is about 1?(2 000-4 000); the mass ratio of adsorbent to waste water is 1?(30-40); and processing time is 2-4 h. Finally, the field tests were done at the optimum technology conditions, which show that the total concentration of Pd and Ag in the waste water below 11 mg/L can be reduced to be less than 1 mg/L.
Key words:
trace precious metals; recovery; agglomeration; adsorption; waste water;
1 Introduction
Because of their special electric conductivity, precious metals are used increasingly in making electronic elements or equipment, and accordingly, the waste water containing precious metals is drained inevitably in the production of precious metal alloy powder. Recovering precious metals from the waste water is not only beneficial to reducing cost, but also can reserve the resources of precious metals and protect the environment. Therefore, it’s of great theoretical and realistic significance to study the new and effective method for recovering precious metals from the waste water[1].
Generally, there are several methods used for the recovery of precious metals from waste water, such as agglomeration, neutralization, electrolysis, oxidation- reduction, extraction, adsorption, ion-exchange, membrane separation, elution and electrodialysis[2-4]. Each method has its own advantages and disadvantages, and may be effective in recovery of precious metals from the common concentration of waste water,but it may become less efficient or even useless when trace precious metal is to be recovered from waste water[2, 5]. Thus in this study, a novel process was put forward to recover precious metals (mainly Pd and Ag) from the waste water generated in the production of the precious metal alloy powder, and a kind of efficient flocculant was chosen by orthogonal tests.
2 Experimental
2.1 Main reagent and equipment
The waste water used was taken from Precious Metal Alloy Powder Producing Company in Guangdong Province, China. The waste water mainly contained precious metals Pd and Ag with total content of 2-11 mg/L and had a very small amount of impurity ions such as sodium ions, calcium ions, and ferrum ions. Preceding experiments show that the influence of these impurity ions on the recovery efficiency of the precious metals can be ignored, and the reason for this may be that these impurity ions without unstable d-electrons can not react with the flocculant of polythiol to form coordinators, and therefore, can not be agglomerated and adsorbed by the pretreated coconut shell activated carbon.
The reagents used in the experiment were analytically pure hydrochloric acid, analytically pure sodium hydroxide, the flocculant of polythiol and the prepared activated carbon.
IB-3 timing constant temperature blender provided by Shanghai Leici New Jing Co. Ltd., ICP-AES manufactured by LEEMAN LABS Inc. in US, were used.
2.2 Experimental fundamental
Because precious metal elements such as Pd and Ag have unpaired and unstable d-electrons, which can produce coordination compound by combining chemical elements or groups that have lone pair of electrons[6-11]. According to the coordination theory, the flocculant with hydrosulfide radical or oxy- can provide a pair of free electrons that can fill in precious metals’ empty atomic orbit, and then form coordination compounds or larger agglomerate particles that may readily bring about sedimentation. Thus, the polythiol was chosen as flocculant through orthogonal experiments.
Besides the advantages of large specific surface areas and a large number of homogeneous pores, the activated carbon can also combine with other elements such as oxygen, hydroxyl to form functional groups of oxygen on its surface. These functional groups have an important influence on the activated carbon’s adsorption capacity and the mechanism of adsorption. The recovery of precious metals from the waste water becomes unefficient when the common activated carbon is used because of trace precious metals and the effect of some base metals existing in wastewater. It’s noted that larger specific surface areas and proper granularity are also important to the adsorption of precious metals, and thus, a kind of pretreated coconut shell activated carbon was used in the experiment.
2.3 Experimental
The experimental procedures were as follows. The pH value of the waste water was adjusted first; secondly, the flocculants were added into the waste water under swift stirring condition, then agglomeration took place; thirdly, the adsorbents were added to the waste water under slow stirring for 2 min, and then, the waste water was rested to make precious metals fully adsorbed and sedimented; and at last, the concentration of precious metals in supernates was analyzed by ICP-AES.
Precious metals display different properties and the results of agglomeration and adsorption are different because the valence of precious metals in the waste water is influenced by the pH value[7-9]. Thus, firstly, the pH value of the waste water should be adjusted to 8-9 in order to get optimum effect of agglomeration and adsorption. The ions or atoms of precious metals existed in the stable form of colloid or solution when their concentrations were quite low, and they couldn’t sedimentate, or be separated from waste water easily. Several chemical and physical methods were used to break down the stable colloid or solution in order to accelerate the sedimentation of precious metal particles[8-9]. The flocculant of polythiol was added to the solution or colloid under swift stirring condition, and the drops of the polythiol in the solution or colloid became much smaller and highly dispersed, which can significantly enlarge mass transfer area and provide much more chance for combining flocculant with precious metals.
The fine particles formed from the ions or atoms of precious metals by agglomeration are in a highly disperse state and their sedimentation rates are quite low. Since a kind of activated carbon with some functional groups on its surface is very effective in the adsorption of some precious metal particles, the activated carbons can not only accelerate the enrichment process of the precious metal particles, but also improve recovery efficiency of precious metals from the waste water. The sediments can be further treated by method of calcination or super-filtration to get free precious metals or the oxides of precious metals.
3 Results and analysis
In order to obtain the recovery efficiency of precious metals Pd and Ag from the waste water by combining of agglomeration and adsorption, the efficiency η of agglomeration and adsorption is defined as
![]() (1)
(1)
where co is original concentration of Pd and Ag in the waste water, mg/L; cs is the concentration of Pd and Ag in the supernate, mg/L.
3.1 Adsorption isothermal line
The adsorption isothermal line of the system was determined by experiment of statically saturated adsorption at constant temperature of 25℃. The adsorption capacity of the system q is calculated by
![]() (2)
(2)
where ![]() is the equilibrium concentration of precious metal in supernate, mg/L; m is the mass of activated carbon adsorbent, g; V is the volume of wastewater sample, L.
is the equilibrium concentration of precious metal in supernate, mg/L; m is the mass of activated carbon adsorbent, g; V is the volume of wastewater sample, L.
Five test samples of the wastewater were taken with the initial concentration co of precious metal of 10.31, 8.01, 6.02, 4.06 and 2.81 mg/L, respectively, and the volume of each sample was 500 mL, and 1 g of the adsorbent was added into the each sample under slow stirring for 10 min, then the samples were held for 4 h, and finally, the supernate of each sample was analyzed for determining the adsorption equilibrium concentration ![]() from which the adsorption capacity q was determined by Eqn.(2). The experimental result is shown in Fig.1, which indicates that the relation between adsorption capacity q and the concentration of precious metal in wastewater is linear in a double logarithmic plot, which can be expressed as Eqn.(3) with a regression coefficient of 0.998 6.
from which the adsorption capacity q was determined by Eqn.(2). The experimental result is shown in Fig.1, which indicates that the relation between adsorption capacity q and the concentration of precious metal in wastewater is linear in a double logarithmic plot, which can be expressed as Eqn.(3) with a regression coefficient of 0.998 6.
![]() or
or ![]() (3)
(3)
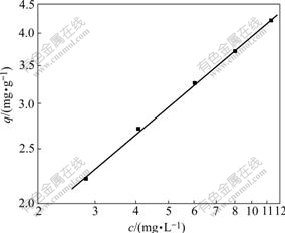
Fig.1 Relation between adsorption capacity (q) and concentration of precious metal in waste water (c)
Eqn.(3) shows that the behavior of the adsorption system conforms to the Freundlich isothermal equation q=acb, and the value of parameter b is in the range of 2-10, which indicates that the polythiol flocculant reacts with precious metals easily[10-13], which is in accordance with the 4th test result in Table 1.
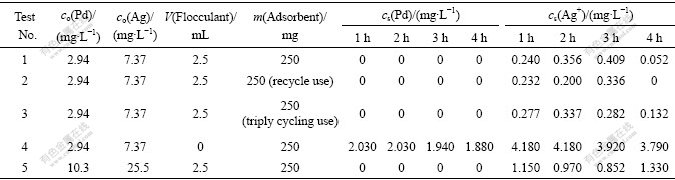
Table 1 Results of field test
3.2 Dynamics of adsorption
By taking 1 g adsorbent and 500 mL waste water of precious metal with initial concentration of 10.31 mg/L,the experiments of adsorption dynamics were done at constant temperature of 25 ℃. The supenates were analyzed every 2 h to determine the adsorption capacity q at different adsorption times. The experimental results are shown in Fig.2, which may be related with Eqn.(4) as follows:
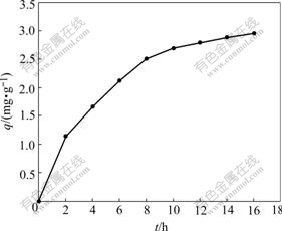
Fig.2 Relation between adsorption capacity and adsorption time
![]() or
or ![]() (4)
(4)
where k is the adsorption rate constant, h-1; R is the ratio of adsorption capacity q to saturated adsorption capacity qe; t is the adsorption time, h.
Eqn.(4) suggests that the relation between (1-R) and t is linear with a slope of k in a single logarithmic plot as shown in Fig.3. The slope k, adsorption rate constant, was calculated as 0.233 4 /h by regressing the experimental data with a relation coefficient of 0.996 2. The result indicates that the process of agglomeration and adsorption is apparent first-order reversal reaction, and mainly limited by the diffusion of precious metal in liquid film[10].
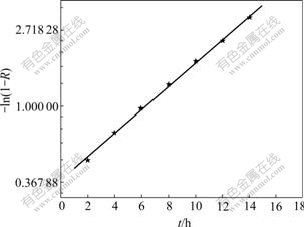
Fig.3 Relation between -ln(1-R) and adsorption time
3.3 Effect of pH value on efficiency of agglomeration and adsorption
0.05 mL flocculant was added into a sample of 200 mL waste water under quick stirring for 2 min, and then the sample was rested till it became turbid. The sample was equally divided into 10 small parts of 20 mL individually; each part was adjusted to different pH values with hydrochloride or sodium hydroxide solution, and 1 g adsorbent was added to the each small part under slow stirring for 2 min; then each part was held for 2-4 h; and at last, the contents of Pd and Ag in the supernates were measured. The effect of pH value on the efficiency of agglomeration and adsorption of precious metal is shown in Fig.4. It shows that the efficiency of agglomeration and adsorption of Ag increases with the rise of the pH value when pH value is less than 6. But when pH value is in the scope of 6-7, the efficiency descends with pH value increasing. The efficiency takes on increasing trend when pH is in the range of 7-9, and reaches the top at pH value of 6 or 9. The agglomeration and adsorption efficiency of Pd increases with the rise of pH value when pH value is below 3. But when pH value is 6-7, the efficiency descends with pH value increasing. The efficiency of Pd also takes on increasing trend when pH value is in the range of 7-9, and reaches peak at the pH value of 3 or 9. The reason for this may be that the oxidation value of the precious metal and the activity of the functional group on the surface of the activated carbon are different in different ranges of pH value, and so, the precious metal Ag and Pd may react with the flocculent polythiol or functional groups on the surface of activated carbon to form different coordination compounds with different solubilities in water[3, 14-15]. The precious metal Ag and Pd may easily react with the flocculent or the functional group, and form insoluble compounds with strong coordination link at individual optimum pH value, and the activity of the polythiol and hydrolysis effect of precious metal coordination compound become weaker at the pH value of 7. Comprehensive results of agglomeration and adsorption of Pd and Ag in the waste water by test show that the best result can be obtained at pH value of 8-9.
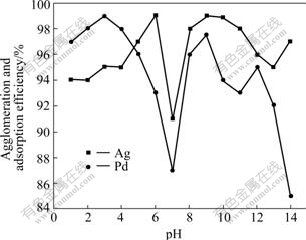
Fig.4 Effect of pH value on agglomeration and adsorption efficiency
3.4 Effect of additive flocculant quantity on agglome- ration and adsorption efficiency
When pH value was in the range of 8-9, 0.025-0.12 mL flocculant was added to 4 samples of 200 mL wastewater respectively under swift stirring condition for 2 min, then a part of 50 mL was taken out from each sample, and 2 g of adsorbent was added to the each parts respectively under slow stirring for 2 min, then was held about 2 h, and at last, the concentrations of the Pd and Ag in the clear solution were measured by ICP-AES. The effects of additive flocculant amount on the efficiency of agglomeration and adsorption are shown in Fig.5. It can be seen that the efficiency of agglomeration and adsorption of precious metals in the waste water does not always increase as additive flocculant quantity rises. The efficiency of agglomeration and adsorption of precious metals is improved with the increase of additive flocculant quantity when the volume ratio of the waste water to flocculant is larger than 4 000? 1. The recovery efficiency of Ag and Pd approaches almost 100% when the additive flocculant quantity is 0.05 mL. The efficiency of agglomeration and adsorption of Pd descends if the additive flocculant quantity exceeds 0.05 mL. The reason for this may be that excessive flocculant exists in the free state and may react disadvantageously with the activated groups on the activated carbon surface, thus, the efficiency decreases. Moreover, excessive flocculant cannot be dispersed sufficiently and floats on the top surface of solution in the free state, which may pollute the environment and increase the recovery cost simultaneously. Thus, the appropriate volume ratio of the flocculant to wastewater is about 1?(2 000-4 000).

Fig.5 Effect of flocculant quantity on agglomeration and adsorption efficiency
3.5 Effect of adsorbent quantity on agglomeration and adsorption efficiency
When pH value was in the optimum range of 8-9, 0.02 mL flocculant was respectively added to each part of 50 mL divided from a 200 mL sample under fast stirring for 2 min, then 0.5, 1.0, 1.5 and 2.5 g adsorbent was added to each part respectively by slow shaking for 1 min, then each share was held for 2-4 h, and at last the contents of Pd and Ag in the supernate were examined. The effect of adsorbent quantity on agglomeration and adsorption efficiency is shown in Fig.6. It indicates that the efficiency of agglomeration and adsorption of precious metals increases as the adsorbent quantity increases when the mass ratio of the adsorbent quantity to waste water is smaller than 1?40, but further increase in adsorbent quantity has no dramatic influence on the efficiency when the mass ratio is larger than 1?30. Thus, the optimum mass ratio of adsorbent to the waste water is 1?(30-40) from the perspectives of both economy and adsorption efficiency.
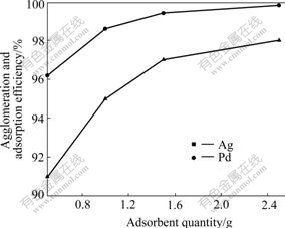
Fig.6 Effect of adsorbent quantity on agglomeration and adsorption efficiency
3.6 Field tests
Field tests were done in the Feng Hua Precious Metal Alloy Powder Producing Company. Two kinds of waste water were fetched from the effluent of the company, and their characteristics are listed in Table 1. A fully mixed sample of 5 L was taken out for each run of field experiment done under the mentioned optimum conditions by the same steps as above. The results of field test are shown in Table 1. The results of field test show that high recovery of the field test is obtained within 4 h under the optimum processing conditions. By comparing the data of test 1 with that of tests 2 and 3 in Table 1, it can be seen that the recovery efficiency of precious metals keeps very good even by recycling use of the adsorbent at least three times. By comparing the 4th test result with other test results in Table 1, it can be found that the flocculant plays an important role in obtaining a high recovery of precious metals from the waste water.
4 Conclusions
1) The technology of reclaiming precious metal from waste water that contains precious metals below 11 mg/L has many advantages such as simpleness, easy operation, less equipment and good economic effects. The efficiency of agglomeration and adsorption of Pd and Ag almost approaches 100%.
2) The experimental data show that Freundlich isothermal equation is suitable for describing features of the system, and the isothermal equation is q=1.35c-2.04, and the apparent first-order adsorption rate constant at 25 ℃ is about 0.233 4 h-1.
3) The technological parameters such as pH value, adsorption time, flocculant quantity and adsorbent quantity have great influence on the efficiency of agglomeration and adsorption of precious metals. The optimum technological conditions are that pH value is 8-9, the volume ratio of the flocculant quantity to the waste water is 1?(2 000-4 000), the mass ratio of the adsorbent quantity to the waste water is 1?(30-40), and the adsorption time is within 4 h.
References
[1] WANG Shi-zhe. An experimental method of recovery of Ag from fixer [J]. North Environment, 2004, 29(1): 53-54. (in Chinese)
[2] ZHOU Fu-chun, XIAN Xue-fu, XU Long-jun. The application and research progress of adsorption on wastewater treatment [J]. China Mining Magazine, 2004, 13(12): 47-49. (in Chinese)
[3] ZHANG Fang-yu, LI Yong-hua. Recovery palladium from wasted catalyst [J]. Precious Metals, 1997, 18(4): 29-30. (in chinese)
[4] ZHANG Yu-lin. Environmental pollution and control[M]. Beijing: Mechanical Industry Press, 1986: 60-90. (in Chinese)
[5] ZHANG Ting-an, DOU Zhi-he. Removal of Ag+ in wastewater with chitosan as flocculation [J]. Journal of Northeastern University: Natural Science, 2006, 27(1): 53-55. (in Chinese)
[6] LI Chuan, GU Guo-bang, LIU Song. Progress in treatment of heavy metals and precious metals in waste-water by TiO2 photocatalysis [J]. Techniques and Equipment for Environmental Pollution Control, 2003, 4(11): 6-11. (in Chinese)
[7] SONG Yu-lin, DONG Zhen-jian. Precious metal chemistry [M]. Shenyang: Liaoning University Press, 1991: 428-544.
[8] LI Ding-xin, ZHANG Yong-li, YUAN Hong-ming. Science of precious metal material [M]. Changsha: Central South University of Technology Press, 1991: 609-642. (in Chinese)
[9] ZHOU Quan-fa. Deep processing and application of precious metal [M]. Beijing: Chemical Industry Press, 2001: 164-166. (in Chinese)
[10] WU Xiang-mei, XIONG Chun-hua, SHU Zeng-ni. Adsorption of silver onto thiol-resin and its mechanism [J]. Journal of Chemical Industry and Engineering, 2003, 54(10): 1466-1468. (in Chinese)
[11] SOMASUNDARA P, RUNKANA V. Selective flocculation of fines [J]. Trans Nonferrous Met Soc China, 2000, 10(s1): 8-11.
[12] CUI Yuan-chen, CHEN Quan, MA Yao-dong. Synthesis and adsorption property of polyethylene-1, 4-dithio-carboxyl piperazine [J]. Chinese Journal of Applied Chemistry, 2002, 19(10): 968-971. (in Chinese)
[13] CHEN Shui-xia, ZENG Han-min. Study on the improvement of the reduction and adsorption capacity of Ag+ activated carbon fibers [J]. Ion Exchange and Adsorption, 2001, 17(5): 316-323. (in Chinese)
[14] HUANG Kun, CHEN Jing, CHEN Yi-ran, ZHAO Jia-chun, LI Qi-wei, YANG Qiu-xu. Recovery of precious metals from spent auto-catalyst by method of pressure alkaline treatment-cyanide leaching [J]. The Chinese Journal of Nonferrous Metals, 2006, 19(2): 363-365. (in Chinese)
[15] JANG Tao. Chemistry of extracting Au [M]. Changsha: Hunan Science and Technique Press, 1998: 182-190. (in Chinese)
Corresponding author: ZOU Hua-sheng; Tel: +86-20-87114539; E-mail: cehszou@scut.edu.cn


