Article ID: 1003-6326(2005)02-0296-04
Effect of silicon on oxidation of Ni-15Al alloy
WU Ying(吴 莹) , NIU Yan(牛 焱), WU Wei-tao( )
)
(State Key Laboratory for Corrosion and Protection, Institute of Metal Research,
Chinese Academy of Sciences, Shenyang 110016, China)
Abstract:
The oxidation of binary Ni-Al alloy containing 15%(mole fraction, the same below if not mentioned) Al (Ni-15Al), and of a ternary alloy with the same Al content but also containing 4% Si (Ni-4Si-15Al) has been studied at 1000℃ under 1.0×105Pa O2 to examine the effect of the addition of Si on the oxidation of Ni-15Al. Oxidation of Ni-15Al produces a duplex scale composed of an outer NiO layer and an inner layer riched in Al2O3. On the contrary, Ni-4Si-15Al forms an external alumina layer directly in contact with the alloy presenting only trace of NiO and the Ni-Al spinel. As a result, the kinetics of Ni-15Al shows a fast initial stage followed by two subsequent parabolic stages with decreasing rate constants, while Ni-4Si-15Al presents essentially a single nearly-parabolic behavior with a rate constant similar to that of the final stage of Ni-15Al. Therefore, the addition of 4% Si significantly reduces the oxidation rate during the initial stage by preventing the formation of Ni-riched scales and promoting an earlier development of an exclusive external alumina layer on the alloy surface.
Key words:
Ni-Si-15Al; ternary alloy; oxidation CLC number: TG146;
Document code: A
1 INTRODUCTION
Chromium, aluminum, and silicon can form satisfactory protective scales on Ni-based alloys. Chromium is expensive and not suitable for use at temperatures above 1000℃ due to the evaporation of CrO3. It has also been well established that the incorporation of Si in many alloy systems has a beneficial effect on their oxidation resistance[1, 2]. In addition, silicon is abundant and cheap. More-over, Si has one of the largest solubility in Ni3Al where it substitutes for Al atoms and thus promotes solution hardening, while it also increases the thermodynamic stability of Ni3Al[3]. The oxidation resistance of Ni-Al alloys was reviewed by Pettit[4] and by Wood and Stott[5], while the oxidation resistance of Ni-Si alloys containing up to 4.97% Si has been the subject of several investigations[6]. These works suggested that a critical aluminum content in the range of approximately 7% to 12.5%(mass fraction) was required for the formation of an exclusive Al2O3 scale on Ni-Al alloys, whereas a critical silicon content between 2.49% and 4.97%(mass fraction) was required for the formation of an exclusively SiO2 scale on Ni-Si alloys. The oxidation resistance of Ni-Si-Al alloys containing 4% Al at 800℃ under (5-9)×10-6 and 1.0×105Pa O2 has been investigated by Yi et al[7], who found that the oxidation rate was increased by the presence of Si during the early stages of oxidation, but was reduced after longer times due to formation of an intermediate layer of NiO and double oxides (NiAl2O4 and Ni2SiO4) between a top NiO layer and a region of internal oxidation. Other two investigations regarded the oxidation of dilute ternary Ni-Si-Al alloys at the oxygen pressure for the Ni/NiO equilibrium, and thus in the absence of an external scale[8, 9], while a further study involved 1.0×105Pa O2[10]. The last works focused on the analysis and interpretation of the kinetics of the coupled internal oxidation of silicon plus aluminum. However, none of the previous papers was concerned with the possible effect of silicon on the formation of alumina scales on binary Ni-Al alloys. The present paper examines the scaling behavior of a binary Ni-15Al and a ternary Ni-4Si-15Al alloys (molar percent, if not specified) in 1.0×105Pa O2 at 1000℃ to clarify the effects of silicon on the oxidation of a Ni-15Al alloy.
2 EXPERIEMNTAL
The alloys prepared by repeated melting appropriate amounts of the high-purity metals (99.999%, mass fraction) have the actual average compositions of Ni-15.2Al and Ni-4.1Si-15.4Al, respectively. The microstructures of both alloys are shown in Fig.1. In agreement with the phase diagram, Ni-15Al presents a mixture of two phases, presenting a light rich and a dark poor in Al phases and Ni-4Si-15Al contains a mixture of two phases, including a light α-phase which is a solid solution of Al and Si in Ni, containing about 12% Al and 4% Si, plus a dark α-phase of Ni3Al containing about 5%Si. The oxidation kinetics were continuously measured by micro-thermobalance at 1000℃ in flowing pure oxygen for 24h. The oxidized samples were examined by means of X-ray diffraction (XRD), scanning electron microscopy (SEM) and an energy-dispersive X-ray microanalysis system (EDX) attached to the SEM.
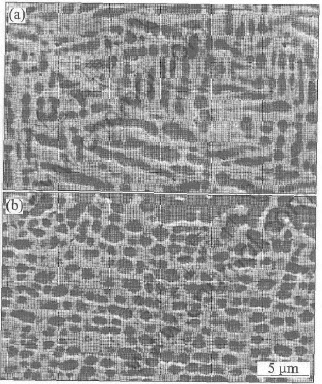
Fig.1 Microstructures of Ni-15Al(a) and 15Al4Si(b)
3 RESULTS AND DISCUSSION
The kinetic curves of the binary Ni-15Al and the ternary Ni-4Si-15Al alloy oxidized at 1000℃ in 1.0×105Pa O2 for 24h are shown in Fig.2(a) as normal plots and in Fig.2(b) as parabolic plots. The mass gains of Ni-4Si-15Al are much lower than those of the binary Ni-15Al alloy. The curve of Ni-15Al alloy can be approximately considered as composed of three subsequent parabolic stages, with average rate constants of about 9.75×10-11g2·cm-4·s-1 for the first 40min, 1.50×10-11g2·cm-4·s-1 from 40min to 400min, and 2.10×10-13g2·cm-4·s-1 from 400min to 24h, respectively. On the contrary, Ni-4Si-15Al presents a nearly parabolic stage with a rate constant equal to 4.75×10-11g2·cm-4·s-1 after a very short initial stage (about 6min) with a rate constant equal to 2.91×10-11g2·cm-4·s-1.
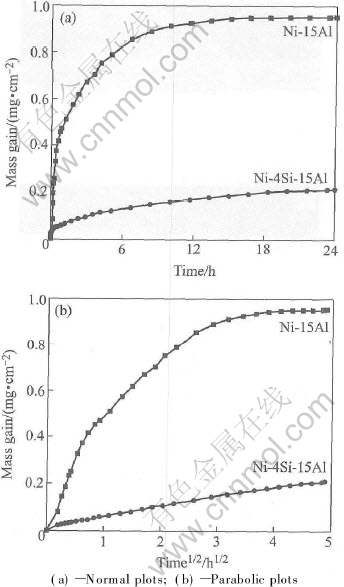
Fig.2 Oxidation kinetics of Ni-15Al and Ni-4Si-15Al oxidized at 1000℃ for 24h in 1.01×105Pa O2
The microstructures of the scales formed on Ni-15Al and Ni-4Si-15Al (shown in Fig.3) are significantly different. The scales formed on Ni-15Al are duplex and contain an outermost layer of NiO and a continuous inner layer composed of a mixed Ni-Al spinel and alumina. On the contrary, the scales formed on Ni-4Si-15Al essentially consist of an external alumina film with only traces NiO and Ni-Al spinel. The preferential Al consumption causes the disappearance of the Al-riched Ni3Al phase in a thick surface layer for both alloys. According to XRD analysis, NiO and the Ni-Al spinel (NiAl2O4) were identified after 24h oxidation on both binary and ternary alloys. In addition, θ-Al2O3, α-Al2O3 and the Ni-Si spinel Ni2SiO4 (although the amounts formed were quite small) were also identified on Ni-4Si-15Al, besides the two metal phases.

Fig.3 Micrographs of cross sections of Ni-15Al(a) and Ni-4Si-15Al(b) oxidized for 24h in 1.01×105Pa O2 at 1000℃
A marked difference exists between the nature of the scales formed on Ni-15Al and Ni-4Si-15Al. When fresh Ni-15Al is exposed to oxygen, NiO, NiAl2O4, and Al2O3 all form together during the fast transient initial stage. Eventually, the scale develops a stable structure, which includes an outermost NiO layer and an inner layer containing a mixture of Ni-Al spinel and α-Al2O3. On the contrary, the ternary alloy Ni-4Si-15Al forms a nearly pure external alumina layer with only small amounts of NiO and spinel. As a result, the initial kinetics of oxidation of Ni-4Si-15Al are considerably slower than those of Ni-15Al. Therefore, the addition of 4% Si reduces significantly the oxidation rate during the initial stage by preventing the formation of Ni-riched oxides and promoting a rapid development of an exclusive alumina layer in the scale.
The effect of silicon observed here for Ni-4Si-15Al, seldom reported in the investigations of the high temperature oxidation of Ni-Si-Al alloys, especially at high oxygen pressures, can be interpreted in the following way. Although Al2O3 may nucleate preferentially over alloy grain boundaries, oxides of all the components in the alloy may nucleate over the remainder of the alloy surface during the initial stage of oxidation[11]. NiO is the fastest growing oxide that can form, thereby accounting for the preponderance of Ni-riched oxides over the surface of Ni-4Si-15Al after the first 6 min of oxidation. The Gibbs free energies of formation of NiO, SiO2 and Al2O3 at 1000℃ are equal to -251, -678 and -845kJ/mol, respectively[12]. Thus, since alumina is much more stable than NiO, as oxidation proceeds and the alloy surface tends to reach a condition of equilibrium with the scale, the binary alloy Ni-15Al develops a continuous layer of alumina, after which the growth of NiO stops with a corresponding strong decrease of the oxidation rate. In the case of the ternary alloy the duration of the initial transient stage and the corresponding formation of the less stable oxides NiO and SiO2 are strongly reduced. Actually, since silicon is much less effective than aluminum in developing rapidly a healing layer of its oxide, SiO2 forms more slowly than Al2O3 and develops less effectively during the early stage[13]. However, its presence is able to prevent almost completely the formation of the least stable oxide NiO, probably by reducing the oxygen pressure prevailing at the alloy/scale interface below the stability of NiO, even simply by becoming enriched at this site with a corresponding reduction of the rate of oxidation. Among the other factors which can contribute to this effect there is a reduction of the size of the particles of the two phases due to the silicon addition, in this way the distance between the Al2O3 nuclei on the grain boundaries is reduced, and hence less time is required for the sideway growth of the nuclei to form a continuous layer of Al2O3. Moreover, the grain boundaries may act as paths for rapid diffusion of Al from the bulk to the surface of the alloy to speed up the process of sideway growth, as proposed by Giggins and Pettit[14, 15] to explain the beneficial effect of a fine grain size on the oxidation resistance of binary Ni-Cr alloys. In addition, silicon may also facilitate the nucleation of the protective scale. Finally, silicon is able to promote the Al diffusion in ternary Ni-Si-Al systems by increasing the effective diffusion coefficient of Al as compared to binary Ni-Al alloys[16]. As a final result, silicon promotes an earlier development of an exclusive alumina layer over the surface of the ternary alloy with respect to the binary Ni-Al alloy with the same Al content.
4 CONCLUSIONS
The addition of silicon has a beneficial effect on the oxidation of a Ni-Al alloy containing 15% Al. The faster diffusion of aluminum in the ternary alloy Ni-4Si-15Al promotes an earlier formation of a protective layer of Al2O3 on the alloy surface during the early stages of oxidation, thereby inhibiting the formation of large amounts of faster growing, Ni-rich oxides. However, after a continuous layer of alumina forms on the surface of the binary alloy, its oxidation rate becomes very similar to that of the ternary alloy.
REFERENCES
[1]Boggs W E. The high-temperature oxidation resistance of iron-silicon-aluminum alloys [J]. Oxid Met, 1976, 10 (4): 277-289.
[2]Guruswamy S, Hirth J P, Powell G W. Oxidation behavior of Fe-Si-Al alloys at 1173-1373K [J]. Oxid Met, 1983, 19 (3-4): 77-98.
[3]Merabtine R, Devaud-Rzepski J, Trichet M F, et al. Two-phase intermetallic alloy Ni3(Al, Si): microstructure and mechanical properties [J]. Intermetallics, 2001, 9: 1015-1020.
[4]Pettit F S. Oxidation mechanisms for nickel-aluminum alloys at temperatures between 900 and 1300℃ [J]. Trans Met Soc AIME, 1967, 239: 1296-1305.
[5]Wood G C, Stott F H. Establishment and breakdown of α-Al2O3 scales on Ni-Al alloys at high temperatures [J]. Brit Corrosion Journal, 1971, 6: 247-256.
[6]Douglass D L, Nanni P, de Asmundis C, et al. The transition form internal oxidation to continuous-film formation during oxidation of dilute Ni-Si alloys [J]. Oxid Met, 1987, 28 (5-6): 309-328.
[7]Yi H C, Shi S Q, Smeltzer W W, et al. Oxidation of γ-Ni-Al-Si alloys at 1073K [J]. Oxid Met, 1995, 43 (1-2): 115-139.
[8]Yi H C, Guan S W, Smeltzer W W, et al. Internaloxidatio of Ni-Al and Ni-Al-Si alloys at the dissociation pressure of NiO [J]. Acta Metall Mater, 1994, 42 (3): 981-990.
[9]Guan S W, Yi H C, Smeltzer W W. Internal oxidation of ternary alloys(PartⅠ)—Kinetics in the absence of an external scale [J]. Oxid Met, 1994, 41 (5-6): 377-387.
[10]Guan S W, Yi H C, Smeltzer W W. Internal oxidation of ternary alloys(PartⅡ)—Kinetics in the presence of an external scale [J]. Oxid Met, 1994, 41 (5-6): 389-400.
[11]Wagner C. Theoretical analysis of the diffusion processed determining the oxidation rate of alloys [J]. J Electrochem Soc, 1952, 99 (10): 369-380.
[12]Kubaschewski O, Alcock C B. Metallurgical Thermochemistry [M]. Oxford: Pergamon Press, 1979. 378-382.
[13]Stott F H, Wood G C, Stringer J. The influence of alloying elements on the development and maintenance of protective scales [J]. Oxid Met, 1995, 44 (1-2): 113-145.
[14]Giggins C S, Pettit F S. Oxidation of Ni-Cr alloys between 800 and 1200℃ [J]. Trans TMS-AIME, 1969, 245: 2495-2507.
[15]Giggins C S, Pettit F S. The effect of alloy grain-size and surface deformation on the selective oxidation of chromium in Ni-Cr alloys at temperatures of 900 and 1000℃ [J]. Trans TMS-AIME, 1969, 245: 2509-2514.
[16]Muralidharan G, Petri M C, Epperson J E, et al. Interaction of Si and Al during interdiffusion in Ni-Al-Si alloys [J]. Scripta Materialia, 1997, 36 (2): 219-225.
Foundation item: Project(50271079) supported by the National Natural Science Foundation of China
Received date: 2004-11-16; Accepted date: 2005-01-18
Correspondence: WU Ying; Tel: +86-24-23915910; Fax: +86-24-23893624; E-mail: ywu@imr.ac.cn


