
![]()

Trans. Nonferrous Met. Soc. China 22(2012) 2535-2540
Synthesis of LiMnPO4/C composite material for lithium ion batteries by sol-gel method
ZHONG Sheng-kui1, 2, 3, WANG You2, LIU Jie-qun1, 2, 3, WANG Jian2
1. Guangxi Key Laboratory of New Energy and Building Energy Saving, Guilin University of Technology,
Guilin 541004, China;
2. College of Chemistry and Bioengineering, Guilin University of Technology, Guilin 541004, China;
3. Shagang School of Iron and Steel, Soochow University, Suzhou 215021, China
Received 9 July 2012; accepted 1 August 2012
Abstract:
The LiMnPO4/C composite material was synthesized via a sol-gel method based on the citric acid. The X-ray diffraction (XRD), scanning electron microscopy (SEM) and electrochemical performance tests were adopted to characterize the properties of LiMnPO4/C. The XRD studies show that the pure olivine phase LiMnPO4 can be obtained at a low temperature of 500 ℃. The SEM analyses illustrate that the citric acid used as the chelating reagent and carbon source can restrain the particle size of LiMnPO4/C well. The LiMnPO4/C sample synthesized at 500 ℃ for 10 h performs the highest initial discharge capacity of 122.6 mA·h/g, retaining 112.4 mA·h/g over 30 cycles at 0.05C rate. The citric acid based sol-gel method is favor to obtain the high electrochemical performance of LiMnPO4/C.
Key words:
lithium-ion battery; cathode material; sol-gel method; LiMnPO4/C; electrochemical performance;
1 Introduction
Polyanion compounds LiMPO4 (M=Mn, Fe, Co and Ni) are considered the promising cathode materials for lithium ion batteries. LiMPO4 (M=Mn, Fe, Co and Ni) cathode materials have more advantages than traditional materials, such as low cost and toxicity [1-3]. The high electrochemical and thermal stabilities of LiMPO4 are attributed to their phosphate structure [4,5]. In these compounds, LiFePO4 has received a wide application. Recently, much attention has been paid to LiMnPO4, because of its high voltage platform of 4.1 V. Compared with LiFePO4, the theoretical energy density of LiMnPO4 is about 1.2 times larger than that of the former [6]. However, LiMnPO4 presents a poor electrochemical performance, which is attributed to difficulty in lithium ion diffusion and low electronic conductivity [7,8]. To these disadvantages of LiMnPO4, one effective approach is to control the particle size. Generally, the appropriate particle size can make lithium ion diffusion easy [9]. However, the carbon coating [10-12] and metal ion doping [13-15] were also used to enhance the properties of LiMnPO4. On the other hand, the electrochemical performance of LiMnPO4 can also be improved by the optimized synthesis route. Taking a example of sol-gel method [16], the precursor is dispersed into the molecular level in the solution and the gel can be obtained during stirring process. Meanwhile, chelating agent is adopted to form the gel in this system. Finally, the sintering temperature is lower compared with traditional solid state route, and the particle size is minimized.
In this work, olivine LiMnPO4/C was synthesized via a sol-gel method based on the citric acid. The citric acid was used as chelating agent and carbon source in this route. As a chelating agent, the citric acid can help precursor to disperse into the molecular level in the solution. During the sintering process, the decomposition of citric acid could minimize the particle size of LiMnPO4/C. The effects of sol-gel route on LiMnPO4/C were measured by TG, XRD, SEM and electrochemical performance test. The optimal synthesis conditions of LiMnPO4/C by sol-gel method could be defined from these results.
2 Experimental
The LiMnPO4/C composite material was synthesized via a sol-gel method based on citric acid. The stiochiometric amounts of Mn(CH3COO)2·4H2O, CH3COOLi·2H2O, H3PO4 and citric acid were dissolved together in distilled water. The PEG400 was added into the aqueous solution. The pH of solution was controlled at 10 by NH3·H2O. With stirring at 60 ℃, the gel was obtained. After drying at 60 ℃ for 48 h, the precursor powder was sintered at 400-600 ℃ for 5-15 h under argon atmosphere.
Powder X-ray diffraction (XRD, X’Pert Pro) using Cu Kα radiation over the 2θ range of 10°-80° with a step size of 0.02° was employed to identify the crystalline phase of the synthesized materials. The particle morphologies of the powders were observed using scanning electron microscope (SEM, JSM-6380LV). The electrochemical characterization was performed using CR2025 coin-type cell. For positive electrode fabrication, the prepared active material LiMnPO4 was mixed with 10% carbon black and 10% polyvinylidene fluoride in N-methyl pyrrolidinone until slurry was obtained. And then the blended slurry was pasted onto an aluminum current collector, and the electrode was dried at 120 ℃ for 4 h in vacuum. The cathode were punched into circular discs with a diameter of 1.2 cm. The test cell consisted of the positive electrode and lithium foil negative electrode separated by a porous polypropylene film, and 1 mol/L LiPF6 in EC+EMC+DMC (1:1:1 in volume) as the electrolyte. The assembly of the cells was carried out in a dry Ar-filled glove box. The cells were charged and discharged over a voltage range of 2.75-4.50 V versus Li/Li+ electrode on a battery tester (BTS-5V3A) at 0.05C rate. The cyclic voltammogram (CV) was investigated by electro-chemical workstation (CHI660A). The CV was tested at a scanning rate of 0.1 mV/s in the voltage range of 2.5-4.5 V.
3 Results and discussion
3.1 TG-DTA curves of precursor
TG-DTA curves for the gel precursor operated at a temperature of 25-1000 ℃ are shown in Fig. 1. As shown in DTA curve, an endothermic peak is observed at 160 ℃, due to the process about thermal decomposition of ammonium salt and organic matter. About 10% of mass loss is observed during the temperature sweep to 160 ℃. It can be seen that the mass loss is about 10% in the second mass loss temperature range of 160-200 ℃. In the temperature range, citric acid begins the decarboxylation reaction, which is an exothermic reaction. As temperature increases to 300 ℃, citric acid begins to burn and release heat. Therefore, a little exothermic peak can be observed at about 300 ℃ in DTA curve. Meanwhile, about 20% of mass loss is yielded during this combustion process. When temperature increases to about 400 ℃, LiMnPO4/C is primarily formed and about 50% mass retains in the final product. So, LiMnPO4/C begins to form at 400 ℃. It can be known that the synthesis temperature of LiMnPO4/C is significantly reduced by sol-gel method.
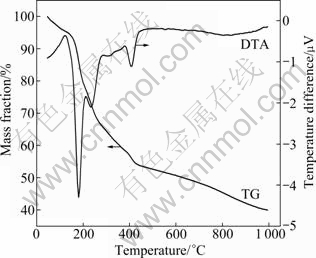
Fig. 1 TG-DTA curves of precursor
3.2 XRD patterns of synthesized LiMnPO4/C samples
X-ray diffraction (XRD) was adopted to investigate the effects of different sintering temperatures and time on crystal structure of LiMnPO4/C. Figure 2 shows the XRD patterns of LiMnPO4/C samples calcined at different temperatures. The single olivine phase in LiMnPO4 can be observed in three XRD patterns, and no impurity peak can be seen. Therefore, it is able to obtain the pure LiMnPO4/C via a sol-gel route at three different temperatures. But, in Fig. 2(a), intensity of diffraction peaks is too low, resulting from the low temperature (e.g. 400 ℃) making a poor crystallinity of LiMnPO4/C. The diffraction peaks of LiMnPO4/C synthesized at 500 ℃ are sharper and narrower compared with others. Therefore, the well crystal structure of LiMnPO4/C can be obtained at 500 ℃. The precursor of LiMnPO4 is dispersed into the molecular level in sol-gel reaction. Then, in the sintering process, these uniform particles can be heated plenty and get a complete reaction.
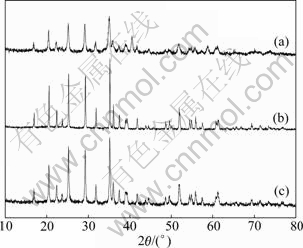
Fig. 2 XRD patterns of LiMnPO4/C samples calcined at different temperatures for 10 h: (a) 400 ℃; (b) 500 ℃; (c) 600 ℃
Figure 3 illustrates that XRD patterns of LiMnPO4/C samples calcined at 500 ℃ for different time. With increasing the calcination time, an apparent increase of intensity of diffraction peaks can be seen in Fig. 3. As shown in Fig. 1(a), intensity of diffraction peaks is low due to the fact that precursor powders cannot get enough time to form well crystal. Over calcination time of 10 h, the diffraction peaks of samples are sharp and narrow. Owing to the extension of sintering time, LiMnPO4/C particles can crystallize perfectly.
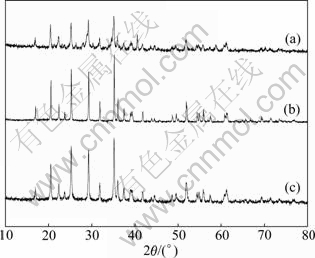
Fig. 3 XRD patterns of LiMnPO4/C samples calcined at 500 ℃ for different time: (a) 5 h; (b) 10 h; (c) 15 h
3.3 SEM images of synthesized LiMnPO4/C samples
Figure 4 shows that SEM images of LiMnPO4/C samples calcined at different temperatures for 10 h. In Fig. 4, LiMnPO4/C particles gradually become larger when sintering temperature increases. This is attributed to that crystal particles grow so fast at high calcination temperature that large-sized particle can be obtained. As seen in Fig. 4(a), LiMnPO4/C particles get incomplete growth at 400 ℃. However, petal-like particles distribute evenly in Fig. 4(b). The decomposition of citric acid at 500 ℃ can improve the morphology of LiMnPO4/C. LiMnPO4/C particles obtained at 600 ℃ are observed so much agglomeration that its conductivity will perform poor. The effects of calcination time on LiMnPO4/C morphology are performed in Fig. 5. When calcination time is 5 h, particle sizes are large in Fig. 5(a). It is attributed to that the short calcination time is hard to form small and uniform particles, while long calcination time is not favor to the conformation of LiMnPO4/C particles. The agglomeration can be observed in Fig. 5(c). However, optimal particles are distributed uniformly as Petal-shaped in Fig. 5(b). In summary, the LiMnPO4/C sample synthesized at 500 ℃ for 10 h has the perfect crystal structure and morphology. The decomposition of citric acid can also control the particle size of LiMnPO4/C. Lithium ion diffusion and conductivity of LiMnPO4 can be enhanced via the sol-gel route based on citric acid.
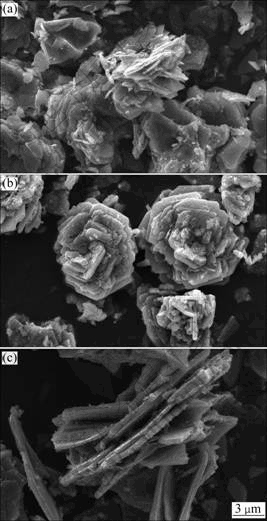
Fig. 4 SEM images of LiMnPO4/C samples calcined zedsing th AN Min, WU Xiang-dong. at different temperatures for 10 h: (a) 400 ℃; (b) 500 ℃; (c) 600 ℃
3.4 Electrochemical characteristics
The first charge-discharge curves of LiMnPO4/C samples synthesized at different temperatures are illustrated in Fig. 6. The LiMnPO4/C samples demonstrate a reversible initial discharge capacity of 110.8 mA·h/g at 400 ℃, 122.6 mA·h/g at 500 ℃ and 114.7 mA·h/g at 600 ℃. The cycling results of three samples are shown in Fig. 7. After 30 cycles, it retained 112.4 mA·h/g at 500 ℃. The capacities of two other samples synthesized at 400 ℃ and 600 ℃ reduced so rapidly, and retained respectively 100.3 mA·h/g and 104.3 mA·h/g after 30 cycles.
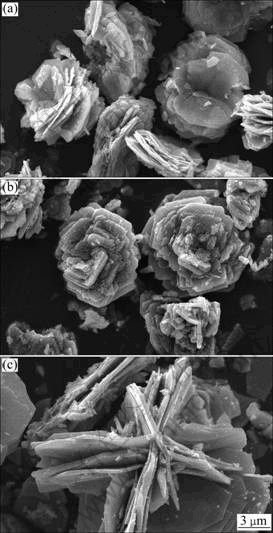
Fig. 5 SEM images of LiMnPO4/C samples calcined at 500 ℃ for different time: (a) 5 h; (b) 10 h; (c) 15 h
The first charge-discharge curves of LiMnPO4/C samples calcined at 500 ℃ with different calcination time are illustrated in Fig. 8. And the results of cycling performance are shown in Fig. 9. The curves present that the good charge-discharge performance is obtained for 10 h, such as the initial discharge capacity of 122.6 mA·g/h and residual capacity of 112.4 mA·h/g after 30 cycles. The LiMnPO4/C sintered for 5 h has the initial discharge capacity of 111.7 mA·g/h and residual capacity of 100.4 mA·h/g after 30 cycles. However, the first discharge capacity of the sample calcined for 15 h is 115.7 mA·h/g, retains at 104.5 mA·h/g after 30 cycles.
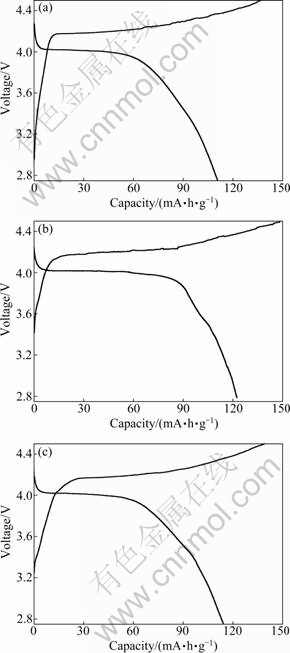
Fig. 6 First charge-discharge curves of LiMnPO4/C samples calcined for 10 h at different temperatures: (a) 400 ℃; (b) 500 ℃; (c) 600 ℃
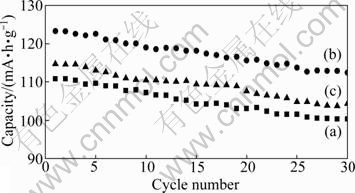
Fig. 7 Electrochemical cycling performance of LiMnPO4/C samples calcined for 10 h at different temperatures: (a) 400 ℃; (b) 500 ℃; (c) 600 ℃
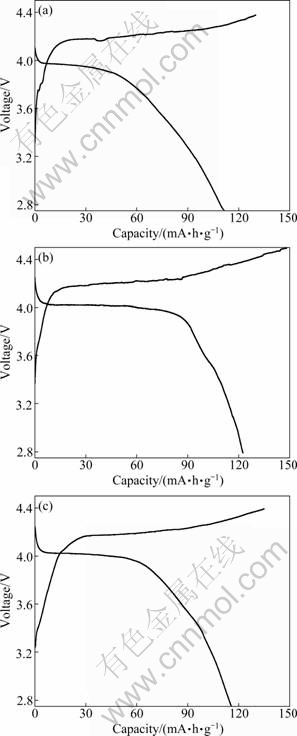
Fig. 8 First charge-discharge curves of LiMnPO4/C samples calcined at 500 ℃ for different time: (a) 5 h; (b) 10 h; (c) 15 h
The CV curves of LiMnPO4/C are illustrated in Fig. 10. The reduction and oxidation peak positions of LiMnPO4/C are located at 3.957 V and 4.362 V, and the difference between reduction and oxidation peak of LiMnPO4/C is 0.405 V. Carbon monoxide released by citric acid in sintering process can maintain the stability of Mn2+. Hence, it reveals that LiMnPO4/C prepared by sol-gel reaction has a good electrochemical reversibility.
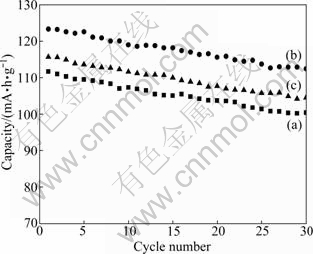
Fig. 9 Electrochemical cycling performance of LiMnPO4/C samples calcined at 500 ℃ for different time: (a) 5 h; (b)10 h; (c) 15 h
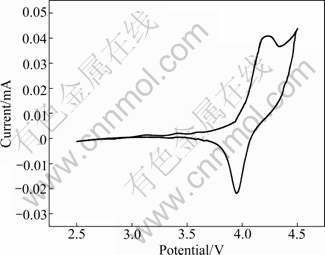
Fig. 10 Cyclic voltammogram curve of LiMnPO4/C
4 Conclusions
1) LiMnPO4/C cathode materials were synthesized via a sol-gel route based on citric acid.
2) LiMnPO4/C synthesized at a low temperature of 500 ℃ for 10 h has an appropriate particle size, while the particle diameter is well-distributed. It performs the best discharge capacity of 122.6 mA·h/g at 0.05C and the retaining capacity of 112.4 mA·h/g over 30 cycles. The CV shows a good electrochemical reversibility of LiMnPO4/C.
3) The conductivity and reversibility of LiMnPO4/C are commendably enhanced, by sol-gel route based on citric acid.
References
[1] PADHI A K, NANJUNDASWAMY K S, GOODENOUGH J B. Phospho-olivines as positive-Electrode materials for rechargeable lithium batteries [J]. J Electrochem Soc, 1997, 144(4): 1188-1194.
[2] YIN Yan-hong, LI Shao-yu, YAN Lin-lin, ZHANG Hui-shuang, YANG Shu-ting. Modified carbothermal reduction method for synthesis of LiFePO4/C composite [J]. Transactions of Nonferrous Metals Society of China, 2012, 22(3): 621-626.
[3] KANG B, CEDER G. Battery materials for ultrafast charging and discharging [J]. Nature, 2009, 458: 190-193.
[4] GOODENOUGH J B, HONG H Y P, KAFALAS J A. Fast Na+-ion transport in skeleton structures [J]. Mater Res Bull, 1976, 11(2): 203-220.
[5] BYKOV A B, CHIRKIN A P, DEMYANETS L N, DORONIN S N, GENKINA E A, IVANOV-SHITS A K., KONDRATYUK I P, MAKSIMOV B A, MELNIKOV O K, MURADYAN L N, SIMONOV V I, TIMOFEEVA V A. Superionic conductors Li3M2(PO4)3 (M=Fe, Sc, Cr): Synthesis, structure and electrophysical properties [J]. Solid State Ionics, 1990, 38(1-2): 31-52.
[6] DOAN N L, TANIGUCHI I. Cathode performance of LiMnPO4/C nanocomposites prepared by a combinationof spray pyrolysis and wet ball-milling followed by heat treatment [J]. J Power Sources, 2011, 196(3): 1399-1408.
[7] DELACOURT C, LAFFONT L, BOUCHET R, WURM C, LERICHE J B, MORCRETTE M, TARASCON J M, MASQUELIER C. Toward understanding of electrical limitations (electronic, ionic) in LiMPO4 (M=Fe, Mn) electrode materials [J]. J Electrochem Soc, 2005, 152(5): 913-921.
[8] YONEMURA M, YAMADA A, TAKEI Y, SONOYAMA N, KANNO R. Comparative kinetic study of olivine LiMPO4 (M= Fe, Mn) [J]. J Electrochem Soc, 2004, 151(9): 1352-1356.
[9] WANG D Y, BUQA H, CROUZET M, DEGHENGHI G, DREZEN T, EXNAR I, KWON N H, MINERS J H, POLETTO L, GR?TZEL M. High-performance, nano-structured LiMnPO4 synthesized via a polyol method [J]. J Power Sources, 2009, 189(1): 624-628.
[10] SHIRATSUCHI T, OKADA S, DOI T, JAMAKI J. Cathodic performance of LiMn1-xMxPO4 (M=Ti, Mg and Zr) annealed in an inert atmosphere [J]. Electrochim Acta, 2009, 54(11): 3145-3151.
[11] DOMINKO R, BELE M, GABERSCEK M, REMSKAR M, HANZEL D, GOUPIL J M, PEJOVNIK S, JAMNIK J. Porous olivine composites synthesized by sol-gel technique [J]. J Power Sources, 2006, 153(2): 274-280.
[12] CHEN G, WILCOX J D, RICHARDSON T J. Improving the performance of lithium manganese phosphate through divalent cation substitution [J]. Electrochem Solid-State Lett, 2008, 11(11): 190-194.
[13] BRAMNIK N N, EHRENBERG H. Precursor-based synthesis and electrochemical performance of LiMnPO4 [J]. J Alloys Compd, 2008, 464(1-2): 259-264.
[14] LI G, AZUMA H, TOHDA M. LiMnPO4 as the cathode for lithium batteries [J]. Electrochem Solid-State Lett, 2002, 5(6): 135-137.
[15] MINAKSHI M, SINGH P, THURGATE S, PRINCE K. Electrochemical behavior of olivine-type LiMnPO4 in aqueous solutions [J]. Electrochem Solid-State Lett, 2006, 9(10): 471-474.
[16] PIANA M, CUSHING B L, GOODENOUGH J B, PENAZZI N. A new promising sol–gel synthesis of phospho-olivines as environmentally friendly cathode materials for Li-ion cells [J]. Solid State Ionics, 2004, 175(1-4): 233-237.
溶胶-凝胶法合成LiMnPO4/C锂离子电池复合材料
钟胜奎1, 2, 3, 王 友2, 刘洁群1, 2, 3, 王 健2
1. 桂林理工大学 广西建筑新能源与节能重点实验室,桂林 541004;
2. 桂林理工大学 化学与生物工程学院,桂林 541004;
3. 苏州大学 沙钢钢铁学院,苏州 215021
摘 要:通过溶胶-凝胶法合成 LiMnPO4/C锂离子电池复合材料,采用XRD、SEM和电化学性能测试对LiMnPO4/C进行性能表征。XRD研究表明,在500 ℃下能够合成得到纯的LiMnPO4;SEM研究表明,柠檬酸作为螯合剂和碳源能有效地抑制LiMnPO4/C颗粒的长大。在500 ℃下烧结 10 h合成的LiMnPO4/C样品的电化学性能最好,首次放电容量为122.6 mA·h/g, 以0.05C倍率循环30次后其容量为112.4 mA·h/g。
关键词:锂离子电池;正极材料;溶胶-凝胶法;LiMnPO4/C;电化学性能
(Edited by LI Xiang-qun)
Foundation item: Project (0991025) supported by Natural Science Foundation of Guangxi, China; Project (51164007) supported by the National Natural Science Foundation of China; Project (201101ZD008) supported by Educational Commission of Guangxi, China
Corresponding author: LIU Jie-qun; Tel: +86-512-67164815; E-mail: ljq@suda.edu.cn
DOI: 10.1016/S1003-6326(11)61497-0
Abstract: The LiMnPO4/C composite material was synthesized via a sol-gel method based on the citric acid. The X-ray diffraction (XRD), scanning electron microscopy (SEM) and electrochemical performance tests were adopted to characterize the properties of LiMnPO4/C. The XRD studies show that the pure olivine phase LiMnPO4 can be obtained at a low temperature of 500 ℃. The SEM analyses illustrate that the citric acid used as the chelating reagent and carbon source can restrain the particle size of LiMnPO4/C well. The LiMnPO4/C sample synthesized at 500 ℃ for 10 h performs the highest initial discharge capacity of 122.6 mA·h/g, retaining 112.4 mA·h/g over 30 cycles at 0.05C rate. The citric acid based sol-gel method is favor to obtain the high electrochemical performance of LiMnPO4/C.


