
Modification of primary Mg2Si in Mg-5Si alloys with Y2O3
ZHENG Na(郑娜), WANG Hui-yuan(王慧远), ZHAO Feng(赵峰),
GU Zhen-hua(顾振华), LI Dong(李栋), JIANG Qi-chuan(姜启川)
Key Laboratory of Automobile Materials of Ministry of Education,
Department of Materials Science and Engineering, Jilin University, Changchun 130025, China
Received 15 July 2007; accepted 10 September 2007
Abstract:
The Y2O3 addition to Mg-5Si alloys has a good modification effect on the primary Mg2Si. With 0.05% or 0.1% (mass fraction) Y2O3 additions, the primary Mg2Si begins to change from coarse dendritic shape (about 100 mm) into small polyhedral shape and therefore the alloys exhibits sub-modified microstructure. With 0.2% Y2O3 addition, most of the primary Mg2Si becomes polyhedral shape and its average size is only 25 mm or less. The Mg-5Si alloy exhibits modified microstructure. In addition, the experiments show that the reaction between Mg and Y2O3 cannot occur in the sintered Mg-6Y2O3 compact; however, the reaction among Mg, Si and Y2O3 can occur in the sintered Mg-5Si-6Y2O3 compact. Apart from the adsorption and poisoning manners, other mechanisms may exist in the modification of Y2O3 addition on the primary Mg2Si.
Key words:
microstructure; solidification; Mg2Si; Y2O3; Mg-5Si alloy;
1 Introduction
Magnesium alloys, the lightest commercial alloys developed so far, have great potential for high performance aerospace and automotive applications[1-3]. In recent years, Mg-Si alloys are more and more attractive since the Mg2Si phase can impede grain boundary sliding at elevated temperature[4]. Furthermore, the intermetallic compound Mg2Si exhibits many excellent properties such as high melting temperature, high hardness, and low thermal expansion coefficient [5-7]. However, under normal cast condition, the primary Mg2Si phase exhibits coarse Chinese script or dendritic shape and the eutectic structures is very brittle, which results in the low ductility and strength of the alloys[5].
Modifying treatment is a very effective method to obtain fine microstructure and improve the mechanical properties of the alloys due to low production cost[8]. Therefore, it is very important to choose an appropriate modifier for the modification of the Mg2Si phase in Mg-Si alloys. Recent studies[9] show that rare earth Y can be used to modify the Mg2Si phase in Mg-high Si alloys. More recently, it has been reported[10] that rare earth Ce can be used for the modification of the primary and eutectic Mg2Si in Al-Si-Mg-Cu alloys and a preferred microstructure can be obtained with 0.4% Ce addition. In addition, rare earth oxide like La2O3, also plays an important role in modifying the primary Si in the Al-20Si alloys[11]. Unfortunately, little work has been carried out on the modification of rare earth oxide in Mg-Si alloys.
In the present work, the modification of Y2O3 on the primary Mg2Si in Mg-high Si alloys was investigated. The aim of the study is to develop a new modifier for the modification of the primary Mg2Si in Mg-high Si alloy.
2 Experimental
Industrially pure Mg ingot (99.89%, mass fraction) and Si (99.95%, mass fraction) were used as starting materials to prepare Mg-5Si alloy. Detailed preparation of Mg-Si alloy was described in our previous study[5]. About 300 g of Mg-5Si alloy was remelted at 800 ℃ in a graphite crucible in an electric resistance furnace under the protection of a mixed gas atmosphere of SF6 (1%, volume fraction) and CO2 (balance). After holding for 10 min, Y2O3 was added to the melts to get the designed composition of Mg-5Si alloys with different Y2O3 contents of 0.05%, 0.1% and 0.2% (mass fraction), respectively. And the melts were manually stirred for about 3 min using a stainless steel impeller and held for 30 min at that temperature. After that the melts were poured into a steel mold preheated at 200 ℃ to produce tabulate samples of 12 mm×120 mm×180 mm. Metallographic samples were cut at the same position of the tabulate samples and were prepared in accordance with standard procedures used for metallographic preparation of metal samples. Then the samples were deeply etched with a mixture of HCl+HNO3 (volume ratio 2?1) alcohol solution for about 6 h.
To investigate the possible reaction among the Mg, Si and Y2O3, the powder blenders of Mg (99.6% purity, about 106 μm), Si (99.5% purity, about 15 μm), and Y2O3 were used to sinter Mg-6Y2O3 and Mg-5Si-6Y2O3 (all in mass fraction, %). The powders were prepared by a ball milling with a ball-to-powder mass ratio of about 4?1 for 6 h, and then cold-isostatically pressed at 50 MPa to form cylindrical compact. Then the compacts were heated at 20 ℃/min to 800 ℃ and held for 30 min under the protection of high purity argon gas.
The microstructure and phase analyses were investigated with scanning electron microscope(SEM) (Model JSM-5310, Japan) equipped with energy- dispersive spectrometer(EDS) (Model Link-Isis, Britain) and X-ray diffraction(XRD) (Model D/Max 2500PC Rigaku, Japan).
3 Results and discussion
Figs.1 and 2 show the SEM micrographs and XRD patterns of the Mg-5Si alloys with 0, 0.05%, 0.1% and 0.2% Y2O3 additions. XRD results show the alloys only consist of Mg and Mg2Si phases. No Y-containing products can be detected by the XRD due to the low content of Y2O3 addition in the alloys. Without Y2O3 addition, the primary Mg2Si exhibits coarse dendrite shape with the size of about 100 μm (Fig.1(a)). With 0.05% or 0.1% Y2O3 additions, the primary Mg2Si begins to change into small polyhedral shape (Figs.1(b) and (c)). Therefore, the alloys exhibit sub-modified microstructure. With 0.2% Y2O3 addition, most of the primary Mg2Si becomes polyhedral shape and its average size is only 25 μm or less, as shown in Fig.1(d). Consequently, the Mg-5Si alloy exhibits modified microstructure. It can be concluded from Fig.1 that Y2O3 has a good modification effect on the primary Mg2Si in Mg-5Si alloys.
In order to study the possible reaction among Mg, Si and Y2O3, the compacts of Mg-6Y2O3 and Mg-5Si-6Y2O3 were sintered at 800 ℃. The reason for that the Y2O3 content is chosen as 6% in the compacts is that the reaction products can be detected by XRD. Fig.3 shows the XRD patterns of the unsintered and sintered compacts. It can be seen from Figs.3(a) and (b) that there are only Mg and Y2O3 phase in both unsintered and sintered Mg-6Y2O3 compacts, indicating that Y2O3 cannot react with Mg in the sintered Mg-6Y2O3 compact. It is clear from Fig.3(c) that there are only Mg, Si and Y2O3 phases in the unsintered Mg-5Si-6Y2O3. However, compared with Fig.3(c), it is interesting to note that the amount of Y2O3 is reduced significantly and the unidentified phase is detected by XRD, which suggests that the reaction among Mg, Si and Y2O3 did occur in the sintered Mg-5Si-6Y2O3 compact. In addition, the Mg2Si and MgO phases are also formed in the compact. It should be mentioned that the formation of MgO (Figs.3(b) and (d)) in the compacts is attributed to the poor protection atmosphere, which agrees with the result of our previous study[12].
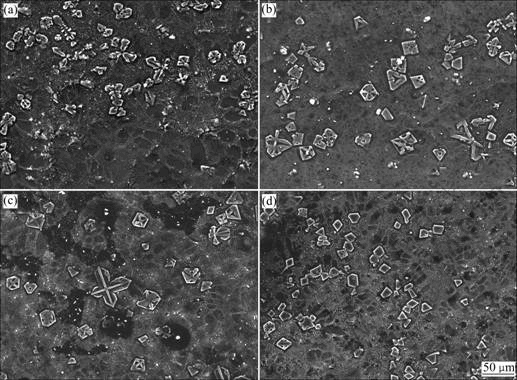
Fig.1 SEM micrographs of Mg-5Si alloys with 0 (a), 0.05% (b), 0.1% (c) and 0.2% (d) Y2O3 additions
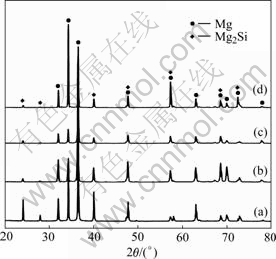
Fig.2 XRD patterns of Mg-5Si alloys with 0 (a), 0.05% (b), 0.1% (c) and 0.2% (d) Y2O3 additions
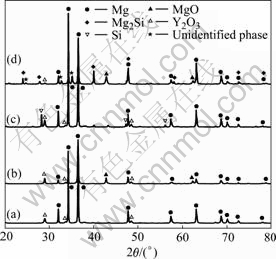
Fig.3 XRD patterns of unsintered (a) and sintered Mg-6Y2O3 (b), and unsintered (c) and sintered Mg-5Si-6Y2O3 (d)
According to the sintering experiments, it is believed that Y may be present in the melts by the decomposition of Y2O3 when Y2O3 is added to the Mg-5Si alloys. During the solidification, the Y atoms are adsorbed on the Mg2Si crystal planes and change the surface energy of the Mg2Si crystals[9], which may effectively poison the growth steps and suppress the preferred growth of the Mg2Si crystals. With 0.05% or 0.1% Y2O3 additions, the Y atoms are insufficient and thus the poisoning effect of Y is not evident, which corresponds to the sub-modified microstructure (Figs.1(b) and (c)). With 0.2% Y2O3 addition, more Y atoms are provided for the adsorption and the poisoning effect. As a result, the primary Mg2Si exhibits modified morphology as a fine polyhedral shape (about 25 μm or less) (Fig.1(d)). Furthermore, it should be noted that Y rare earth just can modify the size rather than the morphology of the primary Mg2Si by the adsorption and poisoning manners; however, the addition of rare earth oxide Y2O3 can modify not only the size but also the morphology of the primary Mg2Si. Therefore, it is possible that other mechanisms exist in the modification of Y2O3 addition on the primary Mg2Si.
4 Conclusions
1) The Y2O3 addition to Mg-5Si alloys has a good modification effect on the primary Mg2Si. With 0.05% or 0.1% Y2O3 addition, the primary Mg2Si begins to change from coarse dendritic shape (about 100 μm) into small polyhedral shape and therefore the alloys exhibits sub-modified microstructure. With 0.2% Y2O3 addition, most of the primary Mg2Si becomes polyhedral shape and its average size is only 25 μm or less. The Mg-5Si alloy exhibits modified microstructure.
2) The reaction between Mg and Y2O3 can not occur in the sintered Mg-6Y2O3 compact; however, the reaction among Mg, Si and Y2O3 can occur in the sintered Mg-5Si-6Y2O3 compact.
3) Apart from the adsorption and poisoning manners, other mechanisms may exist in the modification of Y2O3 addition on the primary Mg2Si.
References
[1] ZHANG J, WANG Y Q, ZHOU B L. Effects of Si content on the microstructure and tensile strength of an in situ Al/Mg2Si composites [J]. J Mater Res, 1999, 14: 68-74.
[2] LIAO L H, ZHANG X Q, WANG H W, QIN X Y, LI X F, MA N H. Influence of Sb on damping capacity and mechanical properties of Mg2Si/Mg-9Al composite materials [J]. J Alloys Compd, 2007, 430: 292-296.
[3] LU L, LAI M Q, HAO M L. Formation of nanocrystalline Mg2Si and Mg2Si dispersion strengthened Mg-Al alloy by mechanical alloying [J]. Nanostruct Mater, 1998, 10: 551-563.
[4] YUAN G Y, LIU M, DING W J, AKIHISA I. Microstructure and mechanical properties of Mg-Zn-Si-based alloys [J]. Mater Sci Eng A, 2003, 357: 314-320.
[5] WANG H Y, JIANG Q C, MA B X, WANG Y, WANG J G, LI J B. Modification of Mg2Si in Mg-Si alloys with K2TiF6, KBF4 and KBF4+K2TiF6 [J]. J Alloys Compd, 2005, 387: 105-108.
[6] WANG L, QIN X Y. The effect of mechanical milling on the formation of nanocrystalline Mg2Si through solid-state reaction [J]. Scripta Mater, 2003, 49: 243-248.
[7] ZHANG J, FAN Z, WANG Y Q, ZHOU B L. Microstructural development of Al-15%Mg2Si in situ composite with mischmetal addition [J]. Mater Sci Eng A, 2000, 281: 104-112.
[8] PAN Y C, LIU X F, YANG H. Microstructural formation in a hypereutectic Mg-Si alloy [J]. Mater Charact, 2005, 55: 241-247.
[9] JIANG Q C, WANG H Y, WANG Y, MA B X, WANG J G. Modification of Mg2Si in Mg-Si alloys with yttrium [J]. Mater Sci Eng A, 2005, 392: 130-135.
[10] QIN Q D, ZHAO Y G, CONG P J, LIANG Y H, ZHOU W. Functionally graded Mg2Si/Al composite produced by an electric arc remelting process [J]. J Alloys Compd, 2006, 389: L1-L4.
[11] XU C L, WANG H Y, YANG Y F, WANG Y, JIANG Q C. Effect of La2O3 in the Al-P-Ti-TiC-La2O3 modifier on primary silicon in hypereutectic Al-Si alloys [J]. J Alloys Compd, 2006, 421: 128-132.
[12] ZHENG N, WANG H Y, WANG W, GU Z H, LI D, JIANG Q C. Invalidation of KBF4 modification on the primary Mg2Si in Mg-Si alloys by Al addition [EB/OL]. J Alloys Compd, 2007, doi:10.1016/j.jallcom.
Foundation item: Project(50501010) supported by the National Natural Science Foundation of China; Project supported by the 985-Automotive Engineering of Jilin University, China
Corresponding author: JIANG Qi-chuan; Tel/Fax: +86-431-85094699; E-mail: jiangqc@mail.jlu.edu.cn
(Edited by PENG Chao-qun)


