
Microstructure and mechanical properties of as-quenched Mg-Gd-Zr alloys
HU Yao-bo1, 2, DENG Juan1, 2, ZHAO Chong1, 2, WANG Jing-feng1, 2, PAN Fu-sheng1, 2
1. College of Materials Science and Engineering, Chongqing University, Chongqing 400044, China;
2. National Engineering Research Center for Magnesium Alloys, Chongqing University, Chongqing 400044, China
Received 23 September 2010; accepted 25 December 2010
Abstract:
Mg-xGd-0.6Zr (x=2, 4, 6, mass fraction) alloys were prepared by semi-continuous casting process. The effects of Gd content on the microstructures and mechanical properties of Mg-xGd-0.6Zr alloys were studied and the solid solution treatment process of Mg-6Gd-0.6Zr alloys was optimized. The microstructures and mechanical properties of all the studied alloys were analyzed by optical microscope, X-ray diffraction, scanning electron microscope equipped with energy dispersive spectroscope and micro-hardness tester. The results show that the grain size slightly decreases with increasing Gd content and there is a close linear relationship between Gd content and micro-hardness of Mg-xGd-0.6Zr alloys. The second phases Mg2Gd and Mg3Gd formed due to non-equilibrium solidification during the casting process can be transformed into equilibrium phase Mg5.05Gd which can dissolve into α-Mg solid solution phase at solution temperature of 460 °C. The optimized solid solution treatment of Mg-6Gd-0.6Zr alloy is (300 °C, 6 h) + (460 °C, 10 h).
Key words:
magnesium alloys; gadolinium; zirconium; microstructure; hardness; heat treatment;
1 Introduction
Magnesium alloys have been intensively investigated in recent years due to their low density, high damping and excellent environmental protection performance. But the low strength of magnesium alloys limits their extensive applications[1-3]. Rare-earths- containing magnesium alloys reveal outstanding mechanical properties at room and elevated temperatures, especially Mg-Gd series alloys which provide excellent cast performance, mechanical properties and corrosion resistance[4]. On the basis of previous investigations, magnesium alloys containing over 10% (mass fraction) Gd show excellent mechanical properties at room and elevate temperatures[5-8]. The strength of Mg-Gd series alloys increases greatly with increasing Gd content, and the change of elongation is contradictory due to their obvious precipitation hardening[9-10]. However, precipitation hardening and solid solution strengthening are two major strengthening mechanisms by the addition of Gd to Mg alloys[11-17]. Few researches were conducted on those alloys containing less than 10% Gd, especially the effect of Gd content less than 10% on solid solution strengthening of Mg-Gd-Zr alloys. The aim of this work is to explore the effect of Gd content on the microstructure and mechanical properties of Mg-xGd-0.6Zr alloys and optimize the solid solution process of Mg-6Gd-0.6Zr.
2 Experimental
The elements of Mg, Gd and Zr in the experimental alloys were supplied in the form of pure Mg (99.8%, mass fraction), Mg-24.51%Gd and Mg-27.85%Zr master alloys. The Mg-xGd-0.6Zr (x=2, 4, 6 mass fraction) alloys were prepared by semi-continuous casting process. The actual compositions of the alloys were determined by XRD-1800 CCDE X-ray fluorescence spectrometer, as shown in Table 1.
As-cast GKx(x=2, 4, 6) alloys were held at 400 °C for 6 h, and at 500 °C for 10 h, then quenched into water to obtain as-quenched GKx alloys. DSC curve of as-cast GK6 alloy was used to determine the solid solution temperature range. GK6 alloy was held at 300 °C for 6 h, and held at 400, 420, 440, 460, 480 and 500 °C for 10 h, respectively, then quenched into water. The effect of Gd content on the microstructures and micro-hardness of Mg-xGd-0.6Zr alloys were studied and the solid solution treatment process of Mg-6Gd- 0.6Zr alloys was optimized. The microstructures and mechanical properties of the alloys were analyzed by optical microscope (OM, MDS produced by OPTEC Company), X-ray diffractometer (XRD, D/max-1200, Cu, 40 kV, 250 mA, 4(°)/min, 10°-90°), scanning electron microscope (SEM, VEGA Ⅱ LMU) equipped with energy dispersive spectroscope (EDS, INCA Energy 350) and micro-hardness tester (HXL-1000AY). In micro-hardness test the load and holding time were 0.49 N and 10 s, respectively.
Table 1 Nominal and actual compositions of Mg-Gd-Zr alloys
3 Results and discussion
3.1 Effects of Gd content on microstructures and mechanical properties of as-quenched Mg-xGd- 0.6Zr alloys
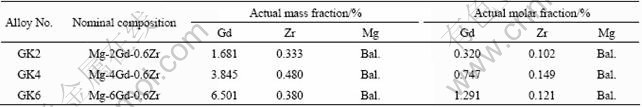
Figure 1 shows the optical microstructures of as-quenched GKx alloys. The mean grain size was measured by linear intercept method. The grain sizes are 54, 48.5, 40.3 μm for GK2, GK4 and GK6 alloys, respectively. With the increase of Gd content, the grain size decreases. According to the Mg-Zr and Mg-Gd phase diagram, it is known that the equilibrium solid solubilities of Zr and Gd in Mg are 0.6% and 22%(mass fraction) at 500 °C. So Zr is hardly solution treated into Mg. Intragranular black phase could be Zr-rich phase in Zr-containing Mg alloys[18], which is not characterized in this work. From the optical microstructure of the alloys, it is seen that there is no phase existing along the grain interface of excluded GK6 alloy, as shown in Fig.1. Figure 2 shows SEM image of as-quenched GK6 alloy. Some white areas appear in matrix along the gain boundary. There is no obvious element-rich phase, as shown in Fig.3. The distribution of Gd in white area is more than that in other matrix.
The XRD patterns (Fig.4) of GKx alloys show that no new phase emerges with increasing the Gd content. α-Mg solid solution phase is the main phase. So Gd has been dissolved into the α-Mg matrix. From the precious EDS results, there is some Zr in the alloys. But the Zr peaks are not marked in Fig.4 because the hexagonal crystal structure of Zr is the same as that of Mg and their peaks coincide with Mg peak. Figure 5 shows Mg peak shifting of as-quenched GKx alloys due to the increase of Gd content[19].
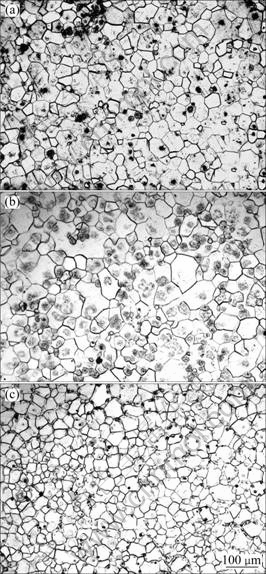
Fig.1 Optical microstructures of as-quenched GK alloys: (a) GK2; (b) GK4; (c) GK6
Figure 6 shows the variation of micro-hardness with Gd content in the as-quenched Mg-Gd-Zr alloys. It seems that the micro-hardness increases monotonically with the increase of Gd content. The hardness (H) is fitted to the Gd content (molar fraction) as
H = 40.16 + 9.30x(Gd) (x(Gd)=0.32-1.29) (1)
where x(Gd) is the Gd molar fraction. Eq.(1) and Fig.6 indicate a near linear increase of hardness with solute concentration, which is very close to the results of Mg-Gd binary alloys. It was reported that the relationship between micro-hardness of Mg-Gd alloys and Gd content in Mg can be expressed as[20]:
H=37+14x(Gd) (x(Gd)=0.49-3.63) (2)
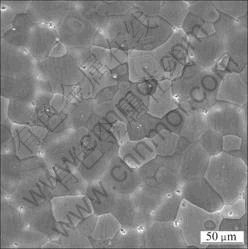
Fig.2 SEM image of as-quenched GK6 alloy
3.2 DSC analyses of as-cast Mg-6Gd-0.6Zr alloy
Figure 7 shows the DSC curve of GK6 alloy. There are three peaks in the curve. The peak at 648 °C should be attributed to Mg melting process, which is an endothermic reaction. From XRD patterns of as-cast GK6 alloys shown in Fig.8, the second phases in as-cast GK6 alloy disappear after solid solution treatment. So in the former two peaks, at least one or two should be the second phases heating reaction peaks. It can be seen that the broadening of peaks is obvious. Maybe a large of residual stress exists in the alloys due to the non-equilibrium solidification during the casting process, or hardening in the intercepting procedure of the studied sample. When the alloys are heated to certain temperature, a large of residual stress is released, which is an exothermic reaction, and leads to partly offset the endothermic peak required by heating reaction of the second phases to make these two peaks broaden and weaken.
There are a large number of Mg2Gd, Mg3Gd phases in as-cast GK6 alloy, but peaks of the new phase Mg5.05Gd appear at low angles and the peaks of Mg2Gd in the 2θ range of 28-29° become weaker after solution treatment at 400 °C. According to the Mg-Gd phase diagram, it can be known that over 53% Gd (mass fraction) is required to satisfy the formation of the second phases Mg2Gd and Mg3Gd at equilibrium solidification. But during the non-equilibrium solidification, the second phases Mg2Gd and Mg3Gd appear. When alloys are heated to certain temperature, the second phases Mg2Gd and Mg3Gd disappear and new equilibrium phases deposit to keep new balance. When the Gd content is 6% (mass fraction), the equilibrium phase is Mg5.05Gd, so the new reactions happen as like-peritectoid action:
α-Mg+Mg2Gd →Mg3Gd
α-Mg+Mg3Gd →Mg5.05Gd
Fig.3 Microstructure (a) and elements distribution of Mg (b), Gd (c) and Zr (d) in as-quenched GK6 alloys
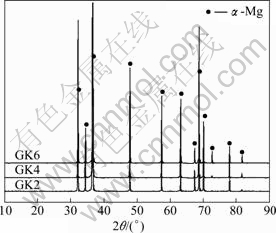
Fig.4 XRD patterns of as-quenched GKx alloys
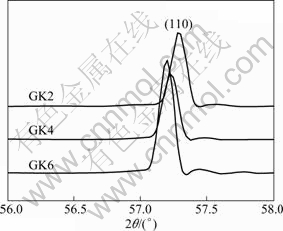
Fig.5 XRD peak shifting of as-quenched GKx due to addition of Gd
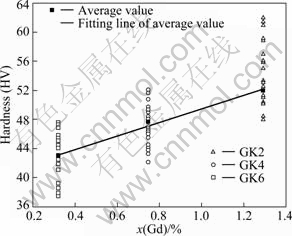
Fig.6 Relationship between hardness and Gd content in as-quenched Mg-Gd-Zr alloys
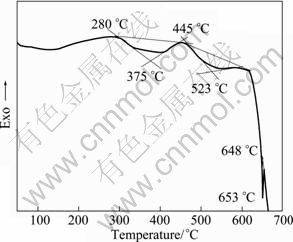
Fig.7 DSC curve of GK6 alloy
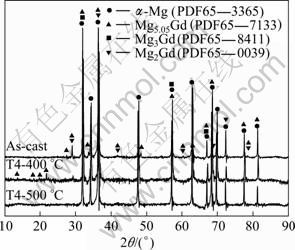
Fig.8 XRD patterns of as-cast, T4-400 °C and T4-500 °C GK6 alloys
Combined with DSC curve of as-cast GK6, the new reaction is an endothermic reaction, which begins at 280 °C, and the peak temperature is 375 °C. The second endothermic peak begins at 445 °C, and the peak temperature is 532 °C. But there is α-Mg solid solution phase, and no other phase exists in alloys after 500 °C solution treatment (as shown in Fig.8).
The second phases Mg2Gd and Mg3Gd formed in non-equilibrium solidification can be transformed into equilibrium phase Mg5.05Gd. However, Mg5.05Gd phase could dissolve into α-Mg solid solution phase at solution temperature 500 °C. So the temperature of 400-500 °C was selected to study the effect of solid solution temperature on microstructure and mechanical properties of alloys and to optimize the solid solution process.
3.3 Effects of solid solution treatment temperature on microstructure and mechanical properties of Mg-6Gd-0.6Zr alloy
Solid solution temperature is one of the most important factors on the second phase atoms dissolving. After solution treatment at 400 °C (as shown in Fig.9(a)), only a small of the second phases in GK6 alloy are dissolved in the matrix due to relatively low temperatures, while most of them distribute densely and continuously along the grain boundary. After solution treatment at 420 °C, the second phase distribution changes from continuous to non-continuous due to some second phase atoms dissolving into Mg matrix (as shown in Fig.9(b)), but the grain size does almost not change. No obvious second phase is found in the microstructure of alloys and the grain size still unconspicuously grows after 440 °C solution treatment (as shown in Fig.9(c)). After treatment at 460 °C (shown in Fig.9(d)), the second phases have been dissolved and the grains have grown slightly. The grains have grown significantly after treatment at 480 °C and 500 °C (shown in Figs.9(e) and (f)) for the temperatures of 480 °C and 500 °C are too high.
In the same solution time, the second phase was dissolved gradually into matrix and the grains grew with the increase of solid solution temperature[21]. In this experiment, the second phases were dissolved thoroughly into matrix at 440 °C and 460 °C and the grains grew negligibly. On one hand, when the solution treatment temperature is too high, the grains will significantly grow up, and even over-burning phenomenon will occur; on the other hand, when the solution treatment temperature is too low, it is difficult for the second phase to dissolve completely into matrix. Therefore, on the condition of making sure second phase fully dissolved, the solution treatment temperature can not be too high.
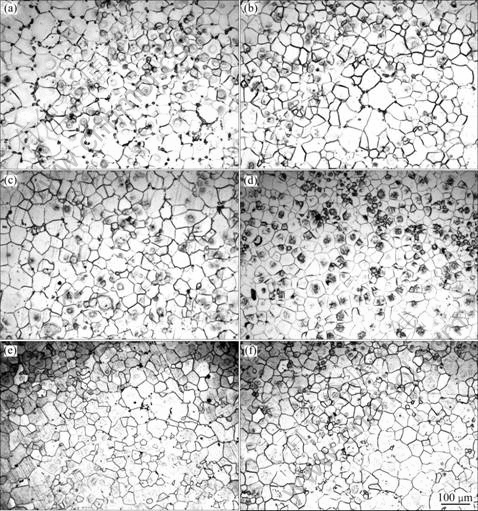
Fig.9 Optical microstructures of as-quenched GK6 after different temperature treatments: (a) 400 °C; (b) 420 °C; (c) 440 °C; (d) 460 °C; (e) 480 °C; (f) 500 °C
Combined with DSC curve of as-cast GK6 alloy (Fig.7) and microstructure of alloys treated at 440 °C and 460 °C (Figs.9(c), (d)), the solid solution process is optimized to be (300 °C, 6 h) + (460 °C, 10 h).
Figure 10 shows the micro-hardness of as-quenched GK6 alloys after different temperature treatments. These micro-hardness values were obtained under the same conditions. It can be seen that the average micro-hardness values increase to peak value at 420 °C and then decrease. The micro-hardness values of alloys treated at 460-500 °C have little difference.
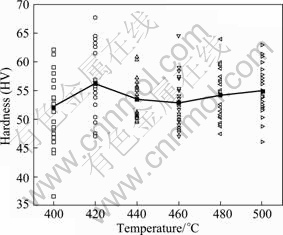
Fig.10 Micro-hardness of as-quenched GK6 after different temperature treatments
The micro-hardness value peak of as-quenched GK6 alloy appears at 420 °C. When the solution temperature is low, only a small part of second phases are dissolved into matrix, and the solid solution strengthening effect is not obvious, so the hardness value is relatively low. But when the solution temperature is too high, it is easy for the second phase to dissolve into matrix, but the grain grows significantly, so the hardness value decreases. While for alloy treated at 420 °C, some second phases atoms are dissolved partly, and some second phases still exist along the grain boundary, so the solid solution strengthening and second strengthening effects improve jointly the micro-hardness of alloy.
4 Conclusions
1) The grain size of as-quenched Mg-xGd-0.6Zr (x=2, 4, 6) alloys decreases, and the hardness value increases with increasing Gd content.
2) There is a linear relationship between Gd content and micro-hardness of alloys. The addition of Zr weakens the solid solution strengthening effects.
3) The second phases Mg2Gd and Mg3Gd formed due to non-equilibrium solidification in the casting process can be transformed into equilibrium phase Mg5.05Gd which can dissolve into α-Mg solid solution phase at solution temperature of 460 °C. The optimized solid solution treatment process is (300 °C, 6 h) + (460 °C, 10 h).
4) The grain sizes of alloys after different temperature treatments increase with increasing solution temperature. Micro-hardness value of the alloy treated at 420 °C is the highest due to the interaction of solid solution strengthening and second strengthening.
References
[1] KAMADO S, IWASAWA S, OHUCHI K, KOJIMA Y, NINOMIYA R. Age hardening characteristics and high temperature strength of Mg-Gd and Mg-Tb alloys [J]. Journal of Japan Institute of Light Metals, 1992, 42(12): 727-733.
[2] IWASAWA S, NEGISHI Y, KAMADO S, KOJIMA Y, NINOMIYA R. Age hardening characterisitics and high temperature tensile properties of Mg-Gd and Mg-Dy alloys [J]. Journal of Japan Institute of Light Metals, 1994, 44(1): 3-8.
[3] LI D J, WANG Q D, BLANDIN J J, SUERY M, DONG J, ZENG X Q. High temperature compressive deformation behavior of an extruded Mg-8Gd-3Y-0.5Zr(wt.%) alloy [J]. Materials Science and Engineering A, 2009, 526(1-2): 150-155.
[4] PENG Q M, WU Y M, FANG D Q, MENG J, WANG L M. Microstructures and properties of Mg-7Gd alloy containing Y [J]. Jounral of Alloys and Compounds, 2007, 430(1-2): 252-256.
[5] YAMADA K, OKUBO Y, SHIONO M, WATANABE H, KAMADO S, KOJIMA Y. Alloy development of high toughness Mg-Gd-Y-Zn-Zr alloys[J]. Materials Transactions, 2006, 47(4): 1066-1070.
[6] PENG Qiu-ming, HOU Xiu-li, WANG Li-dong, WU Yao-ming, CAO Zhan-yi, WANG Li-min. Microstructure and mechanical properties of high performance Mg-Gd based alloys [J]. Materials and Design, 2009, 30(2): 292-296.
[7] WANG Rong, DONG Jie, FAN Li-kun, ZHANG Ping, DING Wen-jiang. Microstructure and mechanical properties of rolled Mg-12Gd-3Y-0.4Zr alloy sheets [J]. Transactions of Nonferrous Metals Society of China, 2008, 18(s1): s189-s193.
[8] ZHENG K Y, DONG J, ZENG X Q, DING W J. Effect of precipitation aging on the fracture behavior of Mg-11Gd-2Nd-0.4Zr cast alloy [J]. Materials Characterization, 2008, 59(7): 857-862.
[9] ANYANWU I A, KAMADO S, KOJIMA Y. Aging characteristics and high temperature tensile properties of Mg-Gd-Y-Zr alloys [J]. Materials Transactions, 2001, 42(7): 1206-1211.
[10] KAWABATA T, MATSUDA K, KAMADO S. HRTEM observation of the precipitates in Mg-Gd-Y-Zr alloy [J]. Materials Science Forum, 2003, 419-422: 303-306.
[11] XIONG Chuang-xiao, ZHANG Xin-ming, DENG Yun-lai, XIAO Yang, DENG Zhen-zhen, CHEN Bu-xiang. Effect of cryogenic treatment on mechanical properties of extruded Mg-Gd-Y-Zr(Mn) alloy [J]. Journal of Central South University of Technology, 2007, 14(3): 305-309.
[12] LIU K, ZHANG J H, ROKHLIN L L, ELKIN M, TANG D X, MENG J. Microstructures and mechanical properties of extruded Mg-8Gd-0.4Zr alloys containing Zn [J]. Materials Science and Engineering A, 2009, 505(1-2): 13-19.
[13] BALASUBRAMANI N, PILLAI U T S, PAI B C. Effect of Zn concentration on the microstructure and phase formation of Mg-5Gd alloy [J]. Journal of Alloys and Compounds, 2008, 460(1-2): L6-L10.
[14] LIU K, ZHANG J H, SU G H, TANG D X, ROKHLIN L L, ELKIN F M, MENG J. Influence of Zn content on the microstructure and mechanical properties of extruded Mg-5Y-4Gd-0.4Zr alloy [J]. Journal of Alloys and Compounds, 2009, 481(1-2): 811-818.
[15] XIAO Yang, ZHANG Xin-ming, CHEN Bu-xiang, DENG Zhen-zhen. Mechanical properties of Mg-9Gd-4Y-0.36Zr alloy [J].Transactions of Nonferrous Metals Society of China, 2006, 16(s3): s1669-s1672.
[16] PENG Qiu-ming, DONG Han-wu, WANG Li-dong, WU Yao-ming, WANG Li-min. Microstructure and mechanical property of Mg-8.31Gd-1.12Dy-0.38Zr alloy [J]. Materials Science and Engineering A, 2008, 477(1-2): 193-197.
[17] YANG Z, LI J P, GAO Y C, LIU T, XIA F, ZENG Z W, LIANG M X. Precipitation process and effect on mechanical properties of Mg-9Gd-3Y-0.6Zn-0.5Zr alloy [J]. Materials Science and Engineering A, 2007, 454-455: 274-280.
[18] ZHANG Zhen-yan, PENG Li-ming, ZENG Xiao-qin, FU Peng-huai, WEN Jiang. Characterization of phases in a Mg-6Gd-4Sm-0.4Zr (wt%) alloy during solution treatment [J]. Materials Characterization, 2009, 60(6): 555-559.
[19] WAN Di-qing, WANG Jin-cheng, YANG Gen-cang. A study of the effect of Y on the mechanical properties, damping properties of high damping Mg-0.6%Zr based alloys [J]. Materials Science and Engineering A, 2009, 517(1-2): 114-117.
[20] GAO L, CHEN R S, HAN E H. Effects of rare-earth elements Gd and Y on the solid solution strengthening of Mg alloys [J]. Journal of Alloys and Compounds, 2009, 481(1-2): 379-384.
[21] GAO Yan, WANG Qu-dong, GU Jin-hai, YANG Zhao, YAN Tong. Behavior of Mg-15Gd-5Y-0.5Zr alloy during solution heat treatment from 500 to 540 °C [J]. Materials Science and Engineering A, 2007, 459(1-2): 117-123.
固溶态Mg-Gd-Zr合金的组织与力学性能
胡耀波1, 2, 邓 娟1, 2, 赵 冲1, 2, 王敬丰1, 2, 潘复生1,2
1. 重庆大学 材料科学与工程学院,重庆 400044;
2. 重庆大学 国家镁合金材料工程技术研究中心,重庆 400044
摘 要:使用半连续铸造法制备Mg-xGd-0.6Zr (x=2, 4, 6, 质量分数) 镁合金,研究不同Gd含量对Mg-xGd-0.6Zr合金组织与力学性能的影响,优化Mg-6Gd-0.6Zr合金的固溶处理工艺。采用光学显微镜、X射线衍射仪、扫描电子显微镜、能谱仪和显微硬度仪对合金的组织和力学性能进行表征。结果表明,随着合金中Gd含量的增加,晶粒尺寸略减小;Mg-xGd-0.6Zr合金的显微硬度与Gd的摩尔浓度呈线性关系。在铸造过程中由非平衡凝固形成的Mg2Gd和Mg3Gd在固溶处理时将转变成Mg5.05Gd平衡相;当固溶温度超过460 °C时,Mg5.05Gd溶解到α-Mg中。Mg-6Gd-0.6Zr合金的优化固溶处理工艺为(300 °C, 6 h) + (460 °C, 10 h)。
关键词:镁合金;钆;锆;组织;硬度;热处理
(Edited by YUAN Sai-qian)
Foundation item: Project (50725413) supported by the National Natural Science Foundation of China; Project (CDJZR10130020) supported by the Fundamental Research Funds for the Central Universities, China
Corresponding author: HU Yao-bo; Tel:+86-13436117593; E-mail:yaobohu@cqu.edu.cn
DOI: 10.1016/S1003-6326(11)60773-5


