
Effect of organic additives with various functional groups on gibbsite morphology
ZENG Ji-shu(曾纪术), YIN Zhou-lan(尹周澜), CHEN Qi-yuan(陈启元)
School of Chemistry and Chemical Engineering,, Central South University, Changsha 410083, China
Received 15 July 2007; accepted 10 September 2007
Abstract:
Gibbsite crystals display large varieties of shapes and sizes when grown from sodium aluminate solution. The usage of gibbsite is partly determined by morphology, therefore, controlling the gibbsite morphology in accordance with usage arrests interests of both merchants and scientists. In present study, the effects of organic additives with various functional groups, oleic acid, 1-octadecanol and stearic acid on gibbsite morphology were investigated at the concentration of 0.1 g/L. Experiments were performed at the temperature of 65 ℃. Samples of gibbsite crystals were obtained from the decomposition of the unseeded synthetic sodium aluminate solution. The morphologies of these gibbsite crystals were examined with SEM, which shows that functional groups influence gibbsite morphology intensively. The morphologies of gibbsite nucleated from the pure solution and solution containing oleic acid, 1-octadecanol and stearic acid are as follows: mosaic gibbsite consisting of various shapes of crystals, radial agglomerate consisting of hexagonal pallets, mosaic and ball like agglomerate consisting of block crystals and loose, radial crystal consisting of irregular tiny nuclei. The facts indicate that it is possible to control gibbsite morphology at the presence of additives with various functional groups. The action mechanism of functional group to morphology was also proposed.
Key words:
1-octadecanol; stearic acid; oleic acid; Gibbsite morphology; functional group;
1 Introduction
Morphology is an important aspect of gibbsite quality, which closely correlates to the characteristics of strength, capacity of adsorption, optical reflectivity and so on. Efforts have been contributed to control alumina trihydrate morphology in recent years. KIM et al[1] concluded that the radial particles can be produced successfully by a careful control of the seed formation, supersaturation and temperature. GR?GORY et al[2] attempted to control particle morphology by stabilizing the pH of sodium aluminate solution at a constant value, and effects of some other parameters on morphology were also investigated. In fact, the growth speed in various direction and agglomeration of gibbsite is sensitive to organic impurities, so it is feasible to control gibbsite morphology by addition of organic additives. Gibbsite is absent of the chamfered faces at the presence of 4-6 mmol/L gluconate and the thinner, plate-like crystals form[3]. Various morphologies of gibbsite can be obtained under the influence of alditols and hydroxy-carboxylic acids[4]. However, there is no report about the exact effect of mono functional group on the gibbsite morphology. In this study, effect of organic additives with various functional groups, oleic acid, 1-octadecanol and stearic acid, on morphology of gibbsite nucleated from pure unseeded sodium aluminate solution were investigated. It was expected to find some clues of the relationship between the organic additive functional group and gibbsite morphology.
2 Experimental
Sodium aluminate solution was prepared by dissolving high purity aluminum(from Xinwei Company, Henan, China) in hot concentrated NaOH solution. Approximately half of the required distilled water was added to the required mass of NaOH in a caustic resistant stainless steel reaction vessel. The mixture was stirred. Upon complete dissolution, the required mass of aluminium metal was then added to the NaOH solution and mixed. After the metal dissolves completely, the liquor was filtered and made up to the required volume.
The caustic alkali concentration of the solution is about 120 g/L, and the caustic mole ratio is 1.30 or so.
The prepared sodium aluminate solution of 900 mL was transferred to an 1 L batch crystallizer, which is a stainless steel vessel with double jacket and a over head stirrer. The solution was bathed with temperature- controlled water and agitated at 150 r/min. After the temperature of the solution reached to 65 ℃, the time was recorded. After 70 h crystallization, the sample was taken out from the crystallizer, filtered, washed and dried at 65 ℃. Morphology of solid sample was observed with a scanning electron microscope (Jeol 6320F).
A crystallization experiment, conducted without any addition of organic molecules, was used as a reference (blank experiment). In other experiments, oleic acid, 1-octadecanol and stearic acid were added, respectively, and the concentration of additive was 0.1 g/L.
3 ResultsThe morphologies of gibbsite grown from the blank solution are shown in Fig.1. The crystals are mosaic agglomerates. Relatively large hexagonal crystals stick together by means of face to face or edge to edge, which constructs skeleton of the agglomerates. A large number of fine particles arrange in the space of agglomerate. Seen from the appearance, the crystals developed with well-formed hexagonal basal faces, prismatic faces and chamfered faces. It is easy to confer that nucleation, growth and agglomeration process take place in the sodium aluminate solution.
The particles precipitated from the solution containing 0.1 g/L 1-octadecanol are solid ball-like mosaic crystals, which are shown in Fig. 2. The agglomerate is mainly consisted of block crystal nearly with the same size and the block crystal is observed to chamfer faces absent but with sharp interfacial boundaries. The formation of block crystal is due to the full growth in the <001> direction. The agglomeration means of crystal is face to face, and fairly perfect ball particle developed by this means.
Interestingly, very large particles developed in the solution at the presence of 0.1 g/L oleic acid, just as the solution containing 1-octadecanol. However, the apparent variations of morphology still exhibit (Fig.3). The crystals are typically radial products, agglomerated by thin hexagonal units and formed very well in edge and corner. The texture of basal faces is fairly smooth while steps and kinks distribute on the prismatic faces. The thin hexagons or tablets are the result of low growth in <001> direction while rapid spread in lateral direction. An important detail should not be ignored, that is, the way that hexagon or tablet sticking together is by small lateral faces instead of larger {001} faces. Some distance always traverse (001) faces, it seems that there is some reason unknown to prevent them sticking together in this direction.
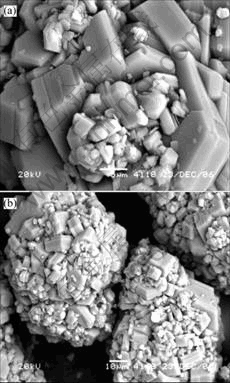
Fig. 1 Morphologies of gibbsite grown in blank solution
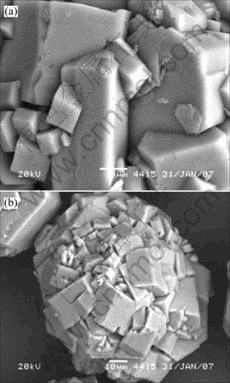
Fig.2 Morphologies of gibbsite grown in solution containing 0.1 g/L 1-octadecanol
The crystal nucleation and growth from the solution containing 0.1 g/L stearic acid are shown in Fig.4. Large particles are loose, radial crystals agglomerated with small poorly formed crystals. A large number of small poorly formed crystals are found to scatter around the large ones. The most possibility of appearance of the mass of small crystal is the second nucleation, which is not observed in the previous sample.

Fig.3 Morphologies of gibbsite grown in solution containing 0.1 g/L oleic acid
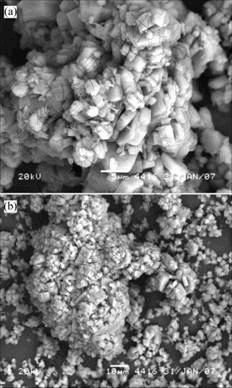
Fig.4 Morphologies of gibbsite grown in solution containing 0.1 g/L stearic acid
4 Discussion
4.1 Change of nucleation with addition of additives
The nucleation and growth rate of gibbsite crystal are known to influence morphology and particle size distribution significantly. For example, rapid precipitation can lead to the formation of amorphism. Generally, the low rate of gibbsite precipitation from caustic sodium aluminate solution is unbearable. To unveil the unusual stability of supersaturated sodium aluminate solution aroused much attention of many investigators. Researchers attempted to make clear the basic structure of aluminate ions, fundamental mechanism of nucleation and the influence of alkali and achieved some progress. Some agreements on this issue were achieved[5-7]. Surface tension of caustic sodium aluminate solution decreases with the addition of surfactant[8], which makes critical nucleation radius decrease and promotes gibbsite nucleation. So neglecting other influence of surfactants, nucleation should be favored, that is, more fine crystals should be observed in the product This agrees quite well with the result that gibbsites grow from the solution containing stearic acid(Fig.4), among which a large number fine crystals are observed. As to the product obtained from the solution containing oleic acid and 1-octadecanol, more factors should be taken into account and will be discussed subsequently.
4.2 Gibbsite faces appearing on experimental morphology
The morphology of natural and industrial gibbsite crystal is pseudohexagonal with {001} basal, {100} and {110} side faces, which agrees fairly well with the present result obtained in blank experiment. SWEEGERS et al[9] investigated that the growth of gibbsite crystals from unseeded sodium aluminate/ sodium hydroxide solutions result in crystals (and agglomerates) with various morphologies, such as lozenge-shaped or hexagonal plates, as well as prisms. He concluded that lozenge-shaped crystals exhibit the basic morphology, single or multiple twinning; the dislocations and presence of impurities lead to the formation of other crystal shapes. The morphology of gibbsite was also predicted on the basis of a complete connected net analysis[10].
Gibbsite growth habit is sensitive to the functional group of organic compound. ISABELLE[11] obtained tabular crystal in the presence of oxalic acid and acicular gibbsite in the presence of DL-malic acid, tartaric acid and tartronic acid. He considered that the adjacent hydroxyl or carboxylic groups of the additive tend to combine with the hydroxyl groups of the gibbsite growth unit and form molecular complex, which can only insert onto specific face of growing crystals. Consequently, a change in gibbsite crystal habit is obtained. Additives used in this investigation contain no adjacent hydroxyl or carboxyl, so we assumed that the hydrophilic-lipophilic ability of surfactant additive also influence gibbsite growth habit intensively.
The length of carbon chains is the same in the oleic acid, 1-octadecanol and stearic acid molecule, which is hydrophobic. The hydrophilic ability of carboxyl in oleic acid and stearic acid is stronger than that of hydroxyl in 1-octadecanol. The unsaturated bond presented in the oleic acid molecule makes it a excellent surfactant. The gibbsite surface is hydrophilic, so the hydrophilic end of additive can easily penetrate through the electrical double-layer of gibbsite surface and be adsorbed at the active site with the hydrophobic carbon chain stretching outside. The strength of hydrophilic end combined with crystal surface determines the crystal development.
Just as other impurities presented in the sodium aluminate solution[12], 1-octadecanol is preferentially adsorbed on the (001) faces of the gibbsite crystals. The hydroxyl combines with crystal surface mildly and does not inhibit crystal growth. On the contrary, 1-octadecanol was found to favor gibbsite crystallization[13]. Crystal develops both in <001> and <100> directions, which leads to the prominent appearance of (001) face and lateral faces. The block-like gibbsite crystal is the result of relative rapid development in <001> direction compared with the blank.
Oleic acid was a preferred collector in floatation of bauxite[14]. It is assumed that oleic acid molecule combines with the exposed Al3+ on the surface of mineral effectively[15], that is to say, the adsorption of oleic acid molecule on gibbsite crystal surface decreases the number of active site available for growth unit, thereby decreases gibbsite growth kinetics. The adsorption mode of oleic acid is similar to that of 1-octadecanol, (001) faces are the primary adsorption planes. The low growth rate in <001> directions compared with lateral directions lead to the formation of thin hexagon crystal, as shown in Fig.3.
Though the addition of stearic acid does not prevent gibbsite nucleation from supersaturated sodium aluminate solution, onset of formation, nuclei further growth are severely inhibited. Therefore, monocrystal develops very slowly in this solution. The mechanism of the stronger inhibit effect of stearic acid than that of oleic acid needs further investigation.
4.3 Agglomeration
Gibbsite agglomeration is influenced by many factors, among which the non-DLVO forces and the precipitation rate of alumina trihydrate were emphasized in present study. According to literature, the agglomeration is generally divided into three steps as follows: 1) particles encounter due to the hydrodynamic of the fluid; 2) aggregates (or phase of association) form, it is a reversible process; 3) aggregates are eventually cemented into a solid particle. The non-DLVO forces involve in steps 1 and 2 while the precipitation rate of alumina trihydrate participates in step 3.
The approach of two particles with hydrated surfaces will generally be hindered by an extra repulsive interaction, distinct from electrical double layer repulsion, that is hydration force. Hydration force was assumed to have an effect on colloid stability, especially at high ionic strength[16]. With the adsorption of surfactant molecules, such as 1-octadecanol and oleic acid, gibbsite crystal surfaces have some degree of hydrophobic character. There is attraction force between hydrophobic surfaces, which can be measured directly[17]. The attraction force promotes the encounter probabilities of gibbsite particle. Furthermore, the adsorption of surfactant molecules on the particles, gives some degree of destabilization (maybe by charge neutralization or by giving opportunity for ‘bridging’ between particles), and collisions between destabilized particles give aggregates, which leads to the formation of enormous sized agglomerates, as shown in Figs. 2 and 3.
The precipitation rate of alumina trihydrate on the surface of gibbsite crystal also influences agglomeration fundamentally. There is a critical amount of freshly precipitated alumina trihydrate to stick aggregates tightly and effectively, otherwise aggregates will break up under the shear stress of fluid or mechanical force. At the presence of 1-octadecano, crystal develops both in basic and lateral faces rapidly, that is, freshly precipitated alumina trihydrate distribute in all unveiled faces. So crystal can agglomerate from any faces, which leads to the formation of solid ball-like agglomerate. As gibbsite growth in <001> direction is inhibited with the adsorption of oleic acid on (001) surfaces, the collisions between (001) faces could not result in effective agglomeration for lacking of enough freshly precipitated alumina trihydrate. Meanwhile, agglomeration in lateral surfaces proceeds. So what we obtained is the large radial crystal agglomerated by small lateral faces. Investigation indicates that gibbsite strength is closely correlated to the stress-state of the overlapped crystals and the strength of two overlapped crystal is directly proportional to the overlapping area of the contacted faces[18]. Obviously, gibbsite prepared in this way is fragile for small overlapping surface area. The precipitated rate of alumina trihydrate on crystal surface is very low at the presence of stearic acid, and the direct result is the formation of radial, small and poorly formed crystals. Maybe superfine gibbsite can be prepared if appropriate amount of stearic acid is adopted in the supersaturated sodium aluminate solution.
5 Conclusions
1) The addition of 1-octadecanol makes crystal grow into solid mosaic ball, and oleic acid makes crystal turn into enormous large, radial, and fragile particle, whereas stearic acid inhibits crystal growth in all directions, resulting in loose, radial and poorly formed crystals.
2) The action mechanism of these additives on gibbsite morphology can be concluded as follows: a) makes the surface tension of sodium aluminate solution decrease and promotes nucleation; b) adsorbs on the gibbsite surfaces and modifies the growth habit of crystal; c) makes the adsorbed surfaces transform from hydrophile to hydrophobia and favors particle encounter.
References
[1] KIM M J, WONG P L M, TRAN T. A study on the precipitation of radial alumina trihydrate[J]. Journal of Crystal Growth , 1997, 178: 360-366.
[2] GREGORY L, VINCENT P, MICHEL F. Controlling particle morphology during growth of bayerite in aluminate solutions[J]. Chem Mater, 2003, 15: 2584-2592.
[3] HELEN W, JOANNE L, HELEN G. Gibbsite crystallization inhibition 1. Effects of sodium gluconate on nucleation, agglomeration and growth[J]. Hydrometallurgy, 2000, 55: 275-288.
[4] HELEN W. Gibbsite crystallization inhibition 2. Comparative effects of selected alditols and hydroxycarboxylic acids[J]. Hydrometallurgy, 2000, 55: 289-309.
[5] POL S, PETER M M, GLENN H. Quantitative determination of an aluminate dimer in concentrated alkaline aluminate solutions by Raman spectroscopy[J]. Dalton Transactions, 2006: 368-375
[6] ANDREA R G. The role of fuzzy interfaces in the nucleation, growth and agglomeration of aluminum hydroxide in concentrated caustic solutions[J]. Progress in Crystal Growth and Characterization of Materials, 2001, 43: 187-220.
[7] YANG Zhong-yu. Production technology of alumina[M]. Second Edition. Beijing: Metallurgical Industrial Press, 1993.
[8] ZHAO Su, BI Shi-wen, YANG Yi-hong, XIE Yan-li. Effect of anionic surfactant on decomposition of sodium aluminate solution[J]. Journal of Northeastern University (Natural Science), 2004, 25(2): 139-141.
[9] SWEEGERS C, VAN Et W J P, MEEKES H, BENNEMA P, HIRALAL I D K, RIJKEBOER A. The impact of twinning on the morphology of γ-Al(OH)3 crystals[J]. Journal of Crystal Growth, 1999, 197: 244-253.
[10] SWEEGERS C, BOERRIGTER S X M, GRIMBERGEN R F P, MEEKES H. Morphology prediction of gibbsite crystals—an explanation for the lozenge-shaped growth morphology[J]. J Phys Chem B, 2002, 106: 1004-1012.
[11] ISABELLE S, STEPHANE V, GERARD P, ROLAND B. The influence of additives on the crystal habit of gibbsite[J]. Journal of Crystal Growth, 1999, 196: 174-180.
[12] BROWN N. Effect of copper ions on the crystallization of bayer aluminium trihydroxide[J]. Journal of Crystal Growth, 1988, 87: 281-286.
[13] YIN Jian-guo, LI Jie, ZHANG Yan-li, CHEN Qi-yuan, YIN Zhou-lan. Effect of monohydroxy-alcohol additives on the seeded agglomeration of sodium aluminate liquors[J]. Light Metals, 2006: 153-157.
[14] ZHANG Guo-fan, FENG Qi-ming, LU Yi-ping, OU Le-ming. Mechanism on diaspore and kaolinite collected by sodium oleate[J]. The Chinese Journal of Nonferrous Metals, 2001, 11(2): 298-301. (in Chinese)
[15] ELIMELECH M, GREGORY J, JIA X, WILLIAMS R A. Particle deposition and aggregation measurement, modeling and simulation[M]. USA: Hardcover, 1995.
[16] HEALY T W, HOMOLA A, JAMES R O. Coagulation of amphoteric latex colloids: reversibility and specific ion effects[J]. Faraday Disc Chem Soc, 1978, 65: 156-163.
[17] ISRAELACHVILI J N, PASHLEY R M. Measurement of the hydrophobic interaction between two hydrophobic surfaces in aqueous electrolyte solutions[J]. Colloid Interface Sci, 1984, 98: 500-514.
[18] LI Wang-xing, HUA Shu-Gui, YIN Zhou-lan, YE Lu-sheng, LIU Ye-xiang. Stress-state analysis on strength of Al(OH)3 grain[J]. The Chinese Journal of Nonferrous Metals, 2005, 15(2): 775-781. (in Chinese)
Foundation item: Project (2005CB623702) supported by the National Basic Research Program of China
Corresponding author: YIN Zhou-lan; E-mail: xhli@mail.csu.edu.cn


