Article ID: 1003-6326(2005)06-1425-04
Synthesis and electrochemical characterization of
layered Li[Ni1/3Co1/3Mn1/3]O2 cathode material
for Li-ion batteries
YU Xiao-yuan(禹筱元)1,2, HU Guo-rong(胡国荣)2,
PENG Zhong-dong(彭忠东)2, XIAO Jin(肖 劲)2, LIU Ye-xiang(刘业翔)2
(1. School of Chemistry and Chemical Engineering, Central South University,
Changsha 410083, China;
2. School of Metallurgical Science and Engineering, Central South University,
Changsha 410083, China)
Abstract:
Layered LiNi1/3Co1/3Mn1/3O2 materials were synthesized using a nickel-cobalt-manganese carbonate precursor obtained by chemical co-precipitation. The [Ni1/3Co1/3Mn1/3]CO3 precursor and the LiNi1/3Co1/3Mn1/3O2 powders were characterized by X-ray diffraction(XRD) and scanning electron micrograph(SEM). The SEM analysis shows that these particles possess uniform and spherical morphology. The electrochemical properties of the LiNi1/3-Co1/3Mn1/3O2 cathode material for rechargeable lithium-ion batteries such as the galvanostatic charge-discharge performance and cyclic voltammetry(CV) were measured. The results show that an initial discharge capacity of 190.29mA·h·g-1 is obtained in the voltage range of 2.5-4.6V and at a current rate of 0.1C at 25℃.The discharge capacity increases linearly with the increase of the upper cut-off voltage limit.
Key words:
lithium-ion batteries; cathode material; layered structure; nickel-cobalt-manganese oxides CLC;
number: TM911.1 Document code: A
1 INTRODUCTION
Due to the high cost of LiCoO2, a commonly used cathode material in commercial rechargeable lithium-ion batteries, much efforts have been made to develop cheaper cathode materials than LiCoO2, LiNiO2 and LiMnO2 have been studied extensively as possible alternatives to LiCoO2[1-4 ]. Stoichiometric LiNiO2 is known to be difficult to synthesize and its multi-phase reaction during electrochemical cycling leads to structural degradation, and layered LiMnO2 has a significant drawback in its crystallographic transformation to spinel structure during cycling[5-7]. Recently, a concept of one-to-one solid solution of LiCoO2, LiNiO2 and LiMnO2, i.e., LiNi1/3Co1/3Mn1/3O2, was adopted to overcome the disadvantage of LiNiO2 and LiMnO2[8-12]. The layered LiNi1/3Co1/3Mn1/3O2 is an attractive cathode material for rechargeable lithium-ion batteries in several aspects. In this research, layered LiNi1/3-Co1/3Mn1/3O2 was prepared using the nickel-cobalt-manganese carbonate precursor, and the electrochemical properties of LiNi1/3Co1/3Mn1/3O2 were investigated.
2 EXPERIMENTAL
LiNi1/3Co1/3Mn1/3O2 powders were synthesized by mixed carbonate method, an aqueous solution of metal nitrates was made with a cation ratio, n(Ni)∶n(Co)∶n(Mn)=1∶1∶1, the precipitation of [Ni1/3Co1/3Mn1/3]CO3 was achieved by slowly dripping the nitrate solution to a NH4HCO3 solution with continuous stirring. The filtrated precipitate was washed with de-ionized water and dried in air, then mixed with stoichiometric amount of Li2CO3 by ball-milling. The mixed powders were heated at 480℃ for 6h and then calcined at 950℃ for 16h in air.
The thermal behavior of the precursor was examined by thermogravimetric analysis(TGA).The powder was characterized by X-ray powder diffraction measurements using a diffractometer PW 1710 with CuKα radiation (Japan). The morphology of sample was observed using scanning electron microscopy (SEM, KYKY 2800, Japan). The electrochemical properties of LiNi1/3Co1/3Mn1/3O2 as cathode materials were evaluated using prototype cell on LAND-2001A battery program-control test system, using a lithium metal foil as the anode and 1mol/L LiPF6 in a 1∶1 solvent of Ethylene carbonate(EC) and Dimethyl carbonate(DMC) as electrolyte. The separator was made from a Celgard 2400 film microporous polypropylene membrane. The cells were assembled in argon gas filled glove-box.
The microelectrode was produced in glove-box with the mixture of the samples and carbon black as the working electrode in ratio of 8∶1 and with the pure-lithium foil as the count-electrode. The cyclic voltammetry curves were measured by Potentiostat/Gallanostat Model (Perkin-Elmer 273A, EG&E).
3 RESULTS AND DISCUSSION
The thermal behavior of the [Ni1/3Co1/3-Mn1/3]CO3 precursor and Li2CO3 was examined by thermogravimetric analysis(TGA). From the TG and DTA results of the precursor, it reveals that below 350℃, there is a mass loss due to the decomposition process of the carbonate compound. The mass loss of the specimens stops at temperatures above 480℃ until to 1080℃ (Fig.1).
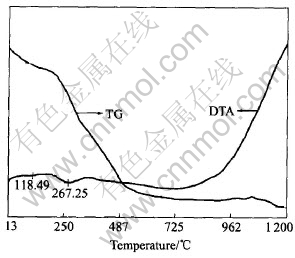
Fig.1 TG and DTA curves of mixture of [Ni1/3Co1/3Mn1/3]CO3 precursor and Li2CO3
The XRD pattern of [Ni1/3Co1/3Mn1/3]CO3 precursor obtained by co-precipitation method is shown in Fig.2(a). Although the XRD pattern of precursor has a low crystallinity, it is found that the precursor has a similar well-defined Ni1/3Co1/3-Mn1/3CO3 hexagonal structure (a=4.52, c=15.6) with no impurity phase, This would be attributed to the homogeneous powder precursor, in which Ni, Co and Mn are uniformly distributed in an atomic scale. The powder X-ray diffraction pattern of LiNi1/3Co1/3Mn1/3O2 finial sample is shown in Fig.2(b). The XRD pattern is well defined and shows the hexagonal doublets (006)/(102) and (108)/(110) a clear splitting, which indicate that they have a high degree of crystallization, good hexagonal ordering and greater layered characteristics. The integrated intensity ratio of the (003) peak to (104) peak(R) in the XRD patterns is shown to be a measure of “cation mixing” and a value of R〈1.2 is an indication of undesirable cation mixing[13, 14]. The ratio of the intensity of the (003) peak to (104) peak of the LiNi1/3Co1/3-Mn1/3O2 sample reported here was calculated to be R=1.42, well above the values reported of undesirable cation mixing. The lattice parameters of LiNi1/3Co1/3Mn1/3O2 are: a=2.866, c=14.262 and match with the values observed by Shaju et al[4] and Yabuuchi et al[15] (a=2.867 and c=14.246), and the c/a ratio is 4.976. The high value of c/a means that the de-intercalation/intercalation of Li+ is more flexible.
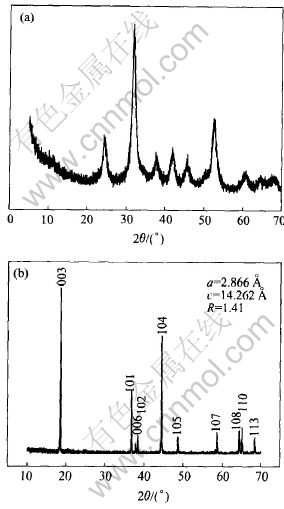
Fig.2 XRD pattern of [Ni1/3Co1/3Mn1/3]CO3 precursor(a) and LiNi1/3Co1/3Mn1/3O2 final sample(b)
The SEM images of precursor and final powders is shown in Fig.3. It can be seen that particles in Ni1/3Co1/3Mn1/3CO3 precursors and the LiNi1/3-Co1/3Mn1/3O2 powders possess spherical morphology. However, the size of LiNi1/3Co1/3Mn1/3O2 particles is more uniform and in the range of 1-2μm.
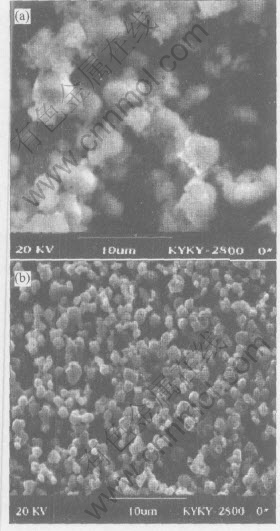
Fig.3 SEM images of [Ni1/3Co1/3Mn1/3]CO3 precursor(a) and LiNi1/3Co1/3Mn1/3O2 final sample(b)
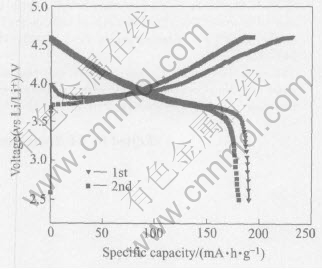
Fig.4 Charge and discharge curves (at 0.1C) of LiNi1/3Co1/3Mn1/3O2 powder at 25℃
Fig.4 shows the charge and discharge curves for the Li/ LiNi1/3Co1/3Mn1/3O2 cell at a current rate of 0.1C in voltage window 2.5-4.6V at room temperature. As seen in Fig.4, the initial discharged capacity of 190.29mA·h·g-1 is obtained. On starting the current, the voltage suddenly increases to about 4V and then slowly decreases to 3.75V and stays along an almost horizontal line at 3.75V, until the charge capacity reaches about 95mA·h·g-1. The slope in the voltage versus capacity curves increase at 95mA·h·g-1 and voltage curves linearly increase until voltage reaches 4.6V, similar to that observed by Yabuuchi and Ohzuku[15] .The irreversible capacity observed in the first cycle is about 40mA·h·g-1.
Fig.5 shows the specific discharge capacity vs number of cycle for Li/ LiNi1/3Co1/3Mn1/3O2 cell at 25℃ at a constant current density of 0.1mA/cm2 in the different voltage range of 2.5-4.3, 2.5-4.4, 2.5-4.5 and 2.5-4.6V. The specific discharge capacity increases linearly with the increase of the upper cut-off voltage limit, the discharge capacities of LiNi1/3Co1/3Mn1/3O2 electrode are 190.29, 172.25,164.27 and 156.12mA·h·g-1, respectively, with good cycleability. The discharge capacities remain at 158.73, 153.59, 149.35 and 146.86mA·h·g-1 after 20 cycles, which are 83.42%, 89.17%, 90.92% and 94.07% of initial capacities, respectively.
Fig.6 shows the cyclic voltammetry curve of the Li/ LiNi1/3Co1/3Mn1/3O2 cell between 2.8V and 4.6V at a scan rate of 0.05mV/s at room
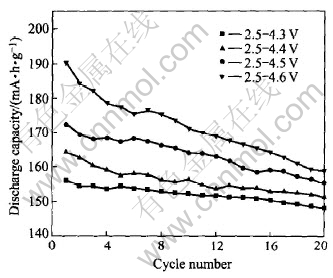
Fig.5 Discharge capacity vs number of cycle for Li/ LiNi1/3Co1/3Mn1/3O2 cell at 0.1C in different voltage range at 25℃
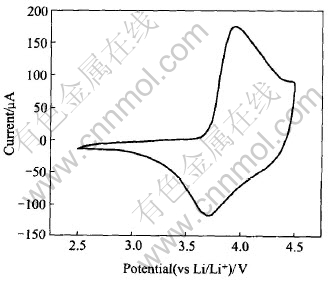
Fig.6 Cyclic voltammetry curve of Li/ LiNi1/3Co1/3Mn1/3O2 cell between 2.5V and 4.6V at scan rate of 0.05mV/s
temperature. As can been seen from Fig.6, the main oxidation peak is observed at 3.9V, while the reduction peak appears at 3.7V, corresponding to Ni2+/4+. The material has a couple of redox peak representing the de-intercalation of Li+ from the initial structure that is observed in a narrow potential range. This implies that the extraction of Li+ occurs easily from an ordered and stabilized layered structure of LiNi1/3Co1/3Mn1/3O2.
4 CONCLUSIONS
The layered LiNi1/3Co1/3Mn1/3O2 was synthesized using a nickel-cobalt-manganese carbonate precursor and characterized by means of XRD, SEM, galvanostatic charge-discharge performance and cyclic voltammetry(CV). The lattice parameters obtained are: a=2.866, and c=14.262. The nicely split (006)/(102) and (108)/(110) peak in the XRD patterns reveal the layered structure of the compound. The initial discharge capacity of 190.29mA·h·g-1 was obtained in the range of 2.5-4.6V and at a current rate of 0.1C at 25℃, and the discharge capacity increases linearly with the increase of the upper cut-off voltage limit. Cyclic voltammetry shows the major redox process at 3.7-3.9V corresponding to Ni2+/4+. The results indicate that the layered LiNi1/3-Co1/3Mn1/3O2 is an attractive cathode material for rechargeable lithium-ion batteries.
REFERENCES
[1]Humg S T , Park H S, Choy J H. Evolution of local structure around manganese in layered LiMnO2 upon chemical and electrochemical delithiation/ relithiation [J]. Chem Mater, 2000, 12: 1818-1826.
[2]Ceder G, Mishra S K. Stability of orthorhombic and monoclinic-layered LiMnO2 [J]. Electrochem Solid State Lett, 1999, 2: 550-552.
[3]Wang G X, Horvat J, Bradhurst D H, et al. Structural physical and electrochemical characterization of LiNixCo1-xO2 solid solutions [J]. J Power Sources, 2000, 85: 279-283.
[4]Kelley T E, Mitchell P H. Lithium manganese oxide-based active material [P]. US 2002031667, 2002-03-14.
[5]Li G H, Iijima Y, Kudo Y, et al. Structural changes of manganese spinel at elevated temperatures [J]. Solid State Ionics, 2002,146: 55-63.
[6]Aral H, Okada S, Sakurai Y, et al. Electrochemical and thermal behavior of LiNi1-zMzO2 (M=Co, Mn, Ti) [J]. J Electrochem Soc, 1997, 144(9): 3117-3125.
[7]Horn Y S, Hackney S A, Armstrong A R, et al. Structural characterization of layered LiMnO2 electrodes by electron diffraction and lattice imaging [J]. J Electrochem Soc, 1999, 146: 2404-2412.
[8]Hwang B J, Tsai Y W, Carlier D, et al. A combined computational experimental study on LiNi1/3Co1/3-Mn1/3O2 [J]. Chem Mater, 2003, 15: 3676-3682.
[9]Kim J M, Chung H T. The first cycle characteristics of Li[Ni1/3Co1/3Mn1/3]O2 charged up to 4.7V [J]. Electrochimica Acta , 2004, 49: 937-944.
[10]Park S H, Yoon C S, Kang S G, et al. Synthesis and structural characterization of layered Li[Ni1/3-Co1/3Mn1/3]O2 cathode materials by ultrasonic spray pyrolysis method [J]. Electrochimica Acta, 2004, 49: 557-563.
[11]Li D Ch, Muta T, Zhang L Q, et al. Effect of synthesis method on the electrochemical performance of LiNi1/3Co1/3Mn1/3O2 [J]. J Power Sources, 2004, 132: 150-155.
[12]Wu Q, Cheng Y, Xu H, et al. Electrochemical properties of nano-sized LiNi1/3Co1/3Mn1/3O2 prepared by sol-gel method [A]. IMLB 12 Meeting [C]. Nara, Japan: The Electrochemical Society, Inc, 2004.
[13]Koyama Y, Tanaka I, Adachi H, et al. Crystal and electronic structures of superstructural Li1-x[Ni1/3-Co1/3Mn1/3]O2 [J] . J Power Sources, 2003, 119-121: 644-648.
[14]Shaju K M, Subba G V, Chowdari B V R. Performance of layered LiNi1/3Co1/3Mn1/3O2 as cathode for Li-ion batteries [J]. Electrochimica Acta, 2002, 48: 145-151.
[15]Yabuuchi N, Ohzuku T. Novel lithium insertion material of LiNi1/3Co1/3Mn1/3O2 for advanced lithium-ion batteries [J]. J Power Sources, 2003, 119-121: 171-174.
(Edited by LI Xiang-qun)
Received date: 2005-03-10; Accepted date: 2005-06-03
Correspondence: YU Xiao-yuan, PhD; Tel: +86-731-8830474; Fax: +86-731-8876454; E-mail address: yxy7021@mail.csu.edu.cn


