DOI:10.19476/j.ysxb.1004.0609.2019.07.18
壳聚糖选择性吸附分离镍、钴新过程
邹小平1, 2,王海北1, 2,王宏岩3, 4,黄 凯4,李 平3
(1. 北京矿冶研究总院,北京 100160;
2. 东北大学 冶金学院,沈阳 110006;
3. 中国科学院 过程工程研究所 湿法冶金清洁生产技术国家工程实验室,北京 100190;
4. 北京科技大学 冶金与生态制造工程学院,北京 100083)
摘 要:
本文以成型壳聚糖为吸附剂进行溶液中Ni-Co的分离,通过Ni2+和Co2+与壳聚糖上氨基的络合能力差异达到Ni2+、Co2+分离的目的。结果表明:利用NaOH与壳聚糖醋酸溶液可制备出粒度约为60 μm的层状成型壳聚糖;成型壳聚糖对Ni2+、Co2+的吸附符合拟二级动力学方程和Freundlich方程;经吸附焓变计算发现成型壳聚糖对Ni2+的吸附为吸热反应,而对Co2+的吸附为放热反应;吸附机理表明,壳聚糖上的氨基和羟基与Ni2+、Co2+发生结合反应。以萃取分离后得到的高Co2+低Ni2+溶液为原料,成型壳聚糖吸附后溶液中Ni2+含量由73×10-6降低至30×10-6,实现杂质的深度脱除。
关键词:
文章编号:1004-0609(2019)-07-1497-12 中图分类号:TF815 文献标志码:A
镍(Ni)、钴(Co)作为重要的战略资源,广泛用在电池、化工、钢铁等行业。Ni、Co的物理和化学性质极其相近,分离困难[1-2]。分离Ni、Co的主要方法有化学沉淀法[3-4]、溶剂萃取法[5-6]等。化学沉淀分离是根据镍化合物与钴化合物的溶度积差异实现分离,包括生成CoOOH的黑镍除钴法[7]、生成CoS沉淀的硫化物沉淀法[8]、生成Co(NH3)63+沉淀的氨水沉淀法[9]。化学沉淀法对于Ni、Co分离有一定效果,但Ni、Co选择性低,操作复杂而漫长,生产效率低下,使其较难在工业上取得应用。目前湿法冶金工业上广泛应用的是溶剂萃取法,其中膦酸类萃取剂P204常用于Ni、Co与Mn、Fe的分离[10-11],P507和Cyanex 272常用于硫酸、氯化体系中Ni、Co的分离[12-16],如李凡等[10]采用P204-磺化煤油体系,研究硫化镍精矿浸出液中杂质Ca、Fe的脱除,结果表明,在试验确定的最佳条件下,Ca、Fe萃取率均达97%以上,较好地实现了浸出液中Ni与杂质的分离。邬建辉等[14]采用钠皂化的P507-磺化煤油体系,研究硫酸镍溶液中Co、Zn、Cu的萃取脱除,经三级逆流萃取,钴的萃取率可达94.17 %,负载有机相用1mol/L硫酸反萃后,Co反萃率高达99.86%,很好地实现了Ni、Co的分离与富集。刘美荣等[16]采用P507-Cyanex272 协同萃取体系,研究钴镍生物浸出液中Ni、Co的分离,考察了初始PH值、油水相比O/A等影响因素,实现了低PH值下Ni、Co的高效分离。其他研究的萃取剂还包括胺类[17-18]和螯合型萃取剂[19-20],如陈松等[21]采用N235-异构醇-磺化煤油萃取体系,研究镍精矿浸出除Fe后溶液中Ni、Co分离回收,结果表明,在高Ni浓度下(>200 g/L),Ni/Co分配比大于5000。李全明等[22]采用丁二酮肟与Co生成螯合物,此螯合物在硫酸铵存在下被乙醇萃取,实现Ni、Co分离。溶剂萃取是Ni、Co分离的主要工艺方法,具有选择性好、回收率高、操作简便的优点,但其中仍存在一定问题,如萃取法固有的溶剂挥发、萃取过程乳化现象、萃取剂损耗等问题导致了萃取过程费用较高,推高了Ni、Co分离的成本。
在热力学上,Ni、Co与NH3络合能力不同,例如Co、Ni与NH3虽然均能结合形成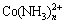 、
、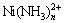 (n<6),但是
(n<6),但是 与
与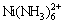 的络合常数分别为4.39和 8.31,络合能力相差较大[22-23]。壳聚糖是一种价廉、安全、无毒的高分子聚合物,其分子中带有游离氨基,由自然界广泛存在的几丁质经过脱乙酰得到。壳聚糖具有吸附特性,已用于吸附Cu、Au、Zn、Cr和Hg[24-28]。这种独特的氨基特性,如能与Ni、Co作用,则有可能实现Ni、Co的选择性分离。而壳聚糖吸附分离Ni、Co研究迄今未见报道。
的络合常数分别为4.39和 8.31,络合能力相差较大[22-23]。壳聚糖是一种价廉、安全、无毒的高分子聚合物,其分子中带有游离氨基,由自然界广泛存在的几丁质经过脱乙酰得到。壳聚糖具有吸附特性,已用于吸附Cu、Au、Zn、Cr和Hg[24-28]。这种独特的氨基特性,如能与Ni、Co作用,则有可能实现Ni、Co的选择性分离。而壳聚糖吸附分离Ni、Co研究迄今未见报道。
本文则以壳聚糖为原料,通过吸附方法分离溶液中的Ni、Co,深入研究Ni、Co的吸附行为、动力学,探究壳聚糖吸附Ni、Co的最佳条件,并探究其吸附机理,对深度分离Ni、Co具有借鉴意义。
1 实验
1.1 实验试剂
本研究所用试剂如下:壳聚糖、氢氧化钠、六水合氯化钴、六水合氯化镍、盐酸,本研究所用试剂均为分析纯且所用水为超纯水。
1.2 成型壳聚糖制备
取3 mL冰醋酸溶于97 mL水中配置成3%醋酸溶液,准确称量3.3 g粉末壳聚糖放入醋酸溶液中,于磁力搅拌下溶解形成胶状溶液。配置1 mol/L 300 mL的NaOH溶液,在200 r/min搅拌转速下,将之前制备好的壳聚糖醋酸溶液缓慢滴加到NaOH溶液中,形成白色颗粒。
将滴加完成后的固液混合物进行过滤,以超纯水将固体洗至中性,再于60 ℃下干燥24 h,经研磨得到成型后壳聚糖吸附剂(CTS)。
1.3 壳聚糖吸附行为探究
1.3.1 吸附行为实验
准确称量5份0.1 g成型壳聚糖置于10 mL Ni2+浓度分别为0.2、0.4、0.6、0.8、1.0 g/L的溶液(pH=5.26, ρ(Co2+)=1g/L)和Co2+浓度分别为0.2、0.4、0.6、0.8、1.0 g/L的溶液(pH=5.26, ρ(Ni2+)=1g/L),分别准确称量0.05、0.075、0.1、0.125、0.15 g 成型壳聚糖置于10 mL的Ni2+、Co2+溶液(ρ0=1 g/L,pH=5.26)中,准确称量5份0.1 g成型壳聚糖置于pH分别为1.32、2.24、3.26、4.42、5.26的Ni2+、Co2+溶液(ρ0=1g/L)中,将上述不同条件下的溶液与成型壳聚糖混合后于303 K下反应 24 h,然后将溶液取出进行Ni2+、Co2+的浓度检测,根据式(1)计算成型壳聚糖对Ni2+、Co2+的吸附量;根据以上吸附量来探究成型壳聚糖的吸附行为。
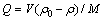 (1)
(1)
式中:ρ0和ρ分别是Ni2+、Co2+的初始浓度和剩余浓度,g/L;V是溶液体积,L;M是成型壳聚糖添加量,g;Q为成型壳聚糖对Ni2+、Co2+的吸附量,mg/g。
1.3.2 吸附动力学实验
准确称量0.15 g 成型壳聚糖置于150 mL Ni2+、Co2+溶液(ρ0=1 g/L,pH=5.3)中,于303 K下反应,分别在5 min、10 min、20 min、35 min、90 min、4h、6h、11h、24 h取样并测定Ni2+、Co2+的浓度,根据式(1)得出不同时间下成型壳聚糖对Ni2+、Co2+的吸附量,探究成型壳聚糖对Ni2+、Co2+的吸附动力学。
1.3.3 吸附热力学实验
准确称量5份0.01 g 成型壳聚糖置于10 mL Ni2+、Co2+溶液(ρ0=1 g/L,pH=5.26)中,分别在303、313、323、333、343 K下恒温振荡反应24 h,过滤后取清液测定Ni2+、Co2+的浓度,根据式(1)得出不同温度下成型壳聚糖对Ni2+、Co2+的吸附量,探索温度与成型壳聚糖对Ni2+、Co2+吸附的关系。
2 结果与分析
2.1 壳聚糖FT-IR检测
图1所示为对壳聚糖成型前后的红外吸收光谱结果。由图1可知,在3430 cm-1出现的主峰来源于O—H和N—H的伸缩振动,而在2919 cm-1和2872 cm-1出现的峰来源于C—H的对称及非对称的伸缩振动,C=O振动主要体现在图中1655 cm-1的峰,N—H的弯曲振动是图中1604 cm-1的峰,894 cm-1的峰来自吡喃环[29]。将两者对比发现,壳聚糖在成型前后红外峰未有明显的变化,说明壳聚糖在成型后保持原有的结构和官能团,预示着成型后的壳聚糖仍能保持较好的吸附性能。
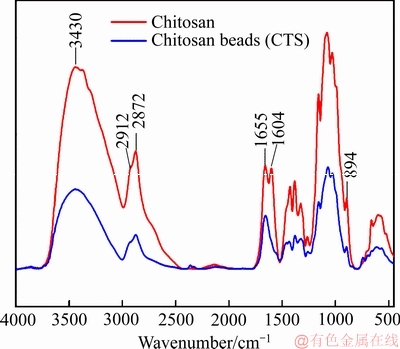
图1 壳聚糖成型前后红外吸收谱图
Fig. 1 Infraredabsorptionspectra of chitosan before and after formation
2.2 壳聚糖SEM检测
对壳聚糖成型前后进行了SEM检测,以探究成型对壳聚糖在形貌上的影响,图2所示为成型前后壳聚糖的SEM像。由图2(a)和(b)可看出,壳聚糖在成型前粒度约在20 μm左右,表面比较疏松,易破碎,且表面有附着较多片层结构的壳聚糖,易脱落。而从图2(c)和(d)可发现,经成型后,壳聚糖由疏松的片层转变为完整的个体,粒度约为60 μm左右,表面也形成一个整体。成型后的壳聚糖不仅粒度得到增加,而且强度得到较大提升,有利于吸附后的壳聚糖分离和循环再生。
2.3 成型壳聚糖(CTS)吸附行为
图3(a)所示为成型壳聚糖对Ni2+、Co2+吸附量与反应时间的关系。由图3(a)可知,成型壳聚糖对Ni2+、Co2+的吸附随着时间的延长而有所增加,对两种离子的吸附速率在逐渐降低,其中成型壳聚糖对Co2+的吸附在达到平衡后开始缓慢降低,对Ni2+的吸附在达到平衡后开始缓慢增加。
成型壳聚糖对Ni2+、Co2+的吸附整体可分为三个阶段:初期阶段(0~1.5 h),中期阶段(1.5~6 h),后期阶段(>6 h)。在初期阶段,成型壳聚糖对Ni2+的吸附较快,吸附量增长较大;到了中期阶段,成型壳聚糖对Ni2+吸附速率减少,吸附量增长较慢;到了6 h后,即进入到后期阶段,此时吸附达到平衡,但吸附量仍有增长的趋势。在吸附反应24 h后,成型壳聚糖对Ni2+、Co2+的吸附量分别达到48.4 mg/g和9.36 mg/g。
与常规吸附行为不同的是,成型壳聚糖对Ni2+、Co2+两种离子的吸附在达到平衡后,对Ni2+的吸附仍出现了增长的趋势,而对Co2+的吸附量在逐渐降低,主要是由于Ni2+、Co2+的竞争性吸附所导致。
在成型壳聚糖吸附未达平衡时,两种离子的吸附都处于上升阶段,吸附官能团还未饱和,而当吸附达到平衡时,吸附官能团已达饱和,Ni2+、Co2+在成型壳聚糖上的竞争性吸附开始影响吸附量。由于壳聚糖上的氨基对Ni2+的结合能力较强,导致Co2+所占据的位点逐渐被Ni2+所取代,从而出现了成型壳聚糖对Ni2+的吸附量在达到吸附平衡后仍然逐渐升高而Co2+的吸附量逐渐降低的现象。
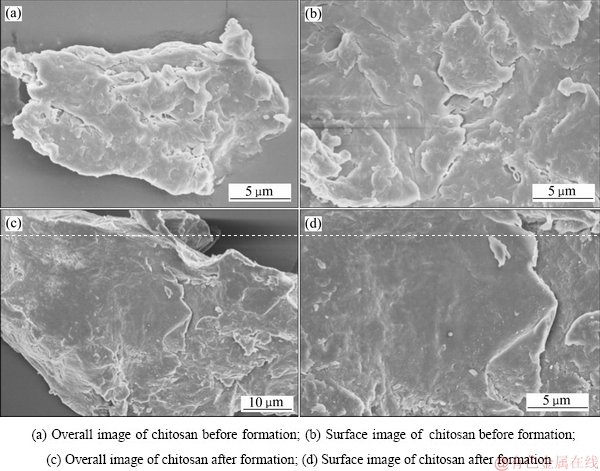
图2 壳聚糖成型前后的SEM像
Fig. 2 SEM images of chitosan before and after formation
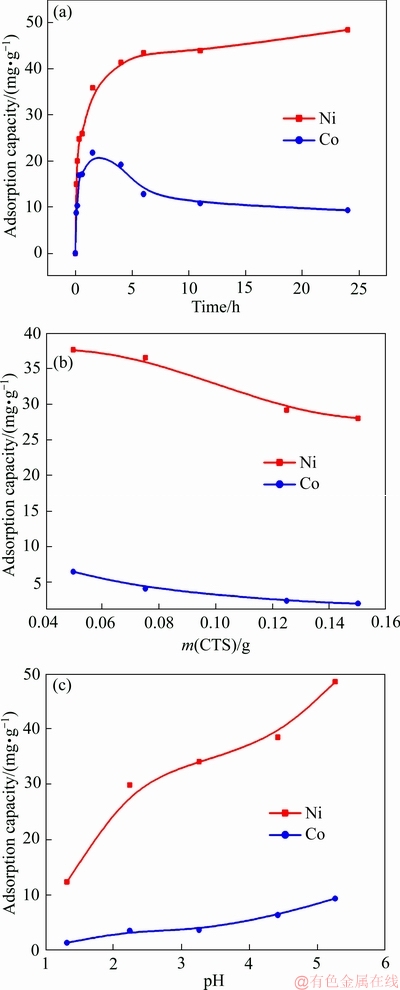
图3 成型壳聚糖对Ni2+和Co2+的吸附量与初始时间、成型壳聚糖加入量、溶液初始pH值的关系
Fig. 3 Adsorption of Ni2+ and Co2+ ions onto CTS as function of time (a), amount of CTS (b) and initial pH (c)
图3(b)所示为成型壳聚糖加入量与其对Ni2+、Co2+吸附量的关系曲线。由图3(b)可知,当成型壳聚糖加入量逐渐增加时,其对Ni2+、Co2+的吸附量逐渐降低。在成型壳聚糖加入量由0.05 g增加至0.15 g情况下,成型壳聚糖对Ni2+的吸附量由37.6 mg/g降低到28 mg/g,对Co2+的吸附量由6.4 mg/g降低到2 mg/g。由于在加入适量成型壳聚糖时,大部分Ni2+和少量Co2+均被吸附至壳聚糖上。而当成型壳聚糖量进一步增加时,尽管未结合的官能团数量在增多,但剩下的Ni2+仍难以与成型壳聚糖的官能团结合;Co2+由于与官能团的结合力远小于Ni2+,更难以被吸附至成型壳聚糖上,从而出现增加成型壳聚糖量不利于其对Ni2+、Co2+吸附的现象[30]。
图3(c)所示为溶液初始pH值与成型壳聚糖对Ni2+、Co2+吸附量的关系曲线。由图3(c)可见,随着溶液初始pH增加,成型壳聚糖对Ni2+、Co2+的吸附量逐渐增加,即提升溶液pH值有利于成型壳聚糖对Ni2+、Co2+的吸附。当溶液pH值从1.32增加到5.26时,成型壳聚糖对Ni2+的吸附量由12.4 mg/g增加到48.4 mg/g,对Co2+的吸附量由1.4 mg/g增加到9.4 mg/g。
由于壳聚糖中含大量—NH2,—NH2在溶液中易与重金属络合,也易与H+结合形成—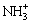 ,主要反应如下:
,主要反应如下:
M2++nRNH2→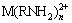 (2)
(2)
H++ nRNH2→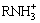 (3)
(3)
在溶液pH值较低情况下,溶液中H+含量较高,壳聚糖中的—NH2更容易与H+结合形成—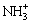 ,进而减少与重金属离子的螯合作用,成型壳聚糖对Ni2+和Co2+的吸附量比较低;在溶液pH值升高时,溶液中的H+含量逐渐减少,越来越多的—NH2与Ni2+、Co2+结合,成型壳聚糖对Ni2+和Co2+的吸附量逐渐升高。在pH值降到一定程度后,壳聚糖中的—NH2与Ni2+、Co2+结合达到饱和,此时成型壳聚糖对Ni2+和Co2+的吸附量达到平衡。
,进而减少与重金属离子的螯合作用,成型壳聚糖对Ni2+和Co2+的吸附量比较低;在溶液pH值升高时,溶液中的H+含量逐渐减少,越来越多的—NH2与Ni2+、Co2+结合,成型壳聚糖对Ni2+和Co2+的吸附量逐渐升高。在pH值降到一定程度后,壳聚糖中的—NH2与Ni2+、Co2+结合达到饱和,此时成型壳聚糖对Ni2+和Co2+的吸附量达到平衡。
2.4 成型壳聚糖(CTS)吸附动力学
本研究采用拟一级动力学方程和拟二级动力学方程[31-33]来对成型壳聚糖吸附Ni2+、Co2+两种离子的吸附动力学数据进行拟合,探究镍钴溶液中成型壳聚糖对两种离子的吸附规律。
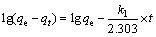 (4)
(4)
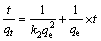 (5)
(5)
式中:qe和qt分别是平衡和t时间内的吸附量,mg/g;k1是拟一级动力学方程的速率常数,min-1;k2是拟二级动力学方程的速率常数,g/(mg·min);t为吸附时间,min。
表1和图4所示为拟一级动力学方程和拟二级动力学方程的拟合结果。如表1所示,拟二级动力学方程对成型壳聚糖吸附Ni2+、Co2+两种离子的拟合相关系数均高于0.98,而且拟合得到Ni2+、Co2+两种离子的平衡吸附量与成型壳聚糖吸附Ni2+、Co2+两种离子的实验吸附量相一致。由于拟一级动力学方程拟合实验数据的相关系数较低,拟合效果不如拟二级动力学方程,而且拟合得到两种离子的平衡吸附量均与实验所得数据相差较大,所以可采用拟二级动力学方程来模拟Ni-Co溶液中成型壳聚糖对Ni2+、Co2+两种离子的吸附过程。
表1 不同离子吸附的拟一级动力学方程和拟二级动力学方程的动力学参数
Table 1 Kinetic parameters of pseudo-first-order model and pseudo-second-order model for different ions adsorption

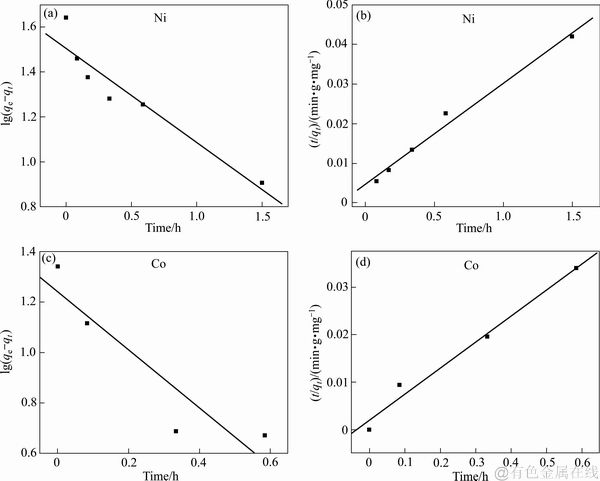
图4 不同离子吸附的拟一级动力学方程和拟二级动力学方程拟合图
Fig. 4 Pseudo-first-order((a), (c)) and pseudo-second-order ((b), (d)) kinetic plots for different ions adsorption
2.5 成型壳聚糖(CTS)吸附等温线
图5所示为不同Ni2+、Co2+浓度下,成型壳聚糖对Ni2+、Co2+的吸附量。由图5可知,在Co2+浓度保持不变情况下,随着Ni2+浓度升高,成型壳聚糖对Ni2+的吸附量在逐渐升高,从8.72 mg/g升至31.05 mg/g,而对Co2+的吸附量在下降,从14.02 mg/g降到12.40 mg/g。同样,在Ni2+浓度保持不变情况下,随着Co2+浓度升高,成型壳聚糖对Co2+的吸附量在逐渐升高,从3.3 mg/g升到16.98 mg/g,而成型壳聚糖对Ni的吸附量有所降低,从31.05 mg/g降低至28.50 mg/g。
本研究采用Langmuir方程和Freundlich方程[34-36]来对成型壳聚糖吸附Ni2+、Co2+两种离子的吸附等温线数据进行拟合,探究镍钴溶液中成型壳聚糖对两种离子的吸附规律,拟合的结果如表2和图6所示,其中ρe代表离子的平衡吸附浓度。。由表2和图6可知,将Langmuir和Freundlich两种方程拟合得到的参数比较发现,成型壳聚糖吸附Ni2+和Co2+过程更符合Freundlich方程。经Langmuir模型拟合得到的最大吸附量与实验所得数据相差较大,且相关系数较低(<0.9),表明成型壳聚糖吸附Ni2+和Co2+过程不符合Ni2+和Co2+的竞争性吸附过程,而经Freundlich模型拟合的相关系数均为0.99,表明成型壳聚糖吸附Ni2+和Co2+过程更符合Freundlich方程,也说明成型壳聚糖对Ni2+和Co2+的吸附为多层吸附,这也在SEM检测中成型壳聚糖的多层结构中证实。
2.6 成型壳聚糖(CTS)吸附焓变
表2 成型壳聚糖吸附Ni2+和Co2+的Langmuir和Freundlich参数
Table 2 Langmuir and Freundlich parameters for Ni2+ and Co2+ ions adsorption onto CTS

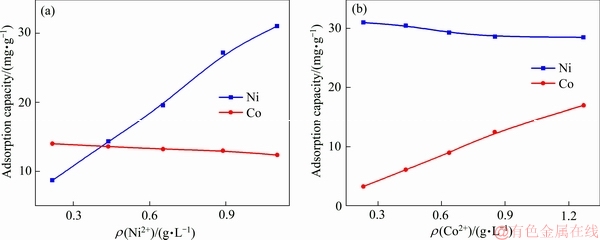
图5 成型壳聚糖对Ni2+和Co2+的吸附与Ni2+、Co2+浓度的关系
Fig. 5 Adsorption of Ni2+ and Co2+ onto CTS as function of Ni2+(a) and Co2+(b) concentrations

图6 Langmuir方程和Freundlich方程对成型壳聚糖吸附Ni2+和Co2+的拟合曲线
Fig. 6 Langmuir adsorption isotherm((a), (c)) and Freundlich adsorption isotherm((b), (d)) for Ni2+ and Co2+ ions adsorption onto CTS
图7所示为成型壳聚糖对Ni2+、Co2+的吸附量与温度的关系。由图7可知,随着温度的升高,成型壳聚糖对Ni2+的吸附量在逐渐升高,而对Co2+的吸附量在逐渐降低,即成型壳聚糖对Ni2+的吸附量从31.00 mg/g升至42.90 mg/g,而对Co2+的吸附量从9.80 mg/g降低至5.56 mg/g,表明升温有利于成型壳聚糖对Ni2+和Co2+的竞争性吸附,高温有利于成型壳聚糖对Ni2+的选择性。
采用Clausius-Clapeyron方程(5)计算成型壳聚糖对Ni2+、Co2+的吸附焓变 (△H,kJ/mol),探究成型壳聚糖对Ni2+、Co2+的吸附热力学规律[37]。
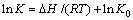 (6)
(6)
式中:K和K0为常数;T为吸附温度,K;R为摩尔气体常数,数值为8.314,J/(K·mol)。
结果显示,Clausius-Clapeyron模型可较好地拟合成型壳聚糖吸附Ni2+和Co2+的吸附,由图8和表3可见,成型壳聚糖对Ni2+的吸附焓变为正值,吸附反应为吸热反应,而成型壳聚糖对Co2+的吸附焓变是负值,这或许与Ni2+、Co2+之间的竞争性吸附有关,高温下成型壳聚糖对Ni2+的选择性较强,从而导致Co2+吸附量的下降。
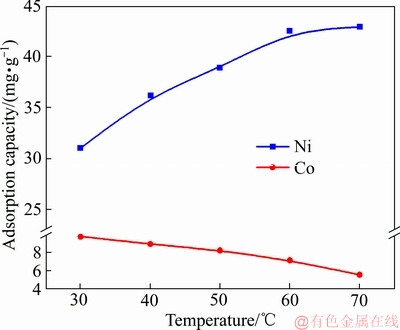
图7 成型壳聚糖对Ni2+、Co2+的吸附量与温度的关系图
Fig. 7 Adsorption of Ni2+ and Co2+ onto CTS as a function of temperature
表3 Clausius-Clapeyron方程对成型壳聚糖吸附Ni2+和Co2+的拟合参数
Table 3 Adsorption thermodynamic parameters obtained from Clausius-Clapeyron equation of Ni2+ and Co2+ ions onto CTS

2.7 成型壳聚糖在高钴低镍溶液中的应用
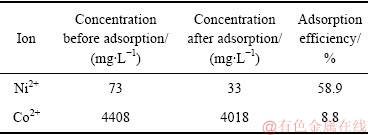
基于上述探索结果,本研究针对Ni-Co传统萃取工艺所得到的高钴低镍溶液进行Ni2+的深度除杂应用进行探究,将2 g成型壳聚糖与20 mL高钴低镍溶液(ρ(Co2+)=4408 mg/L,ρ(Ni2+)=73 mg/L)进行混合吸附,反应24 h后取清液分析除杂效果,结果如表4所示。从表4中可以发现,成型壳聚糖在高钴低镍溶液中可较好地降低Ni2+的含量,在损失8.8% Co2+的情况下,可将溶液中的Ni2+降低58.9%,从而降低Ni2+对溶液指标的影响。成型壳聚糖吸附将高钴低镍溶液的镍钴比由萃取工艺的60:1提高到121:1,使钴溶液进一步纯化。
表4 吸附前后溶液中Ni2+和Co2+成分变化
Table 4 Concentration variations of Ni2+ and Co2+ before and after adsorption
2.8 成型壳聚糖对Ni2+和Co2+的吸附机理探究
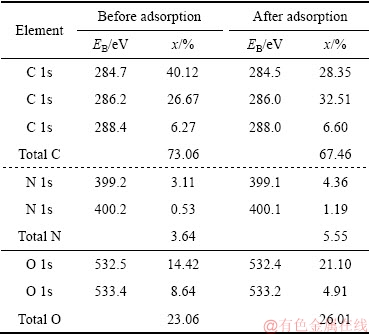
采用XPS分析成型壳聚糖吸附前后的样品的表面价态和表面结构,探究成型壳聚糖对Ni2+、Co2+的选择性吸附机理。图8所示为成型壳聚糖吸附前后的扫描结果。由图8可知,成型壳聚糖吸附前后都能存在着出现在284.7、399.2和532.5 eV的特征峰,这些峰可分别归结为C 1s、N 1s和O 1s。在吸附后的成型壳聚糖检测结果中,可观察到有少量Ni和Co的特征峰,经比较发现Ni的特征峰要强于Co的特征峰,表明Ni2+-Co2+溶液中成型壳聚糖对Ni2+和Co2+的吸附存在竞争性和选择性,且Ni2+的竞争性强于Co2+的。为了进一步探究Ni2+和Co2+的吸附机理,本研究对吸附后的成型壳聚糖中N 1s、O 1s、Co 2p1/2、Co 2p3/2、Ni 2p1/2和Ni 2p3/2进行了检测,其结果如图9和表5所示。在399.2和400.2 eV出现的特征峰与—NH2和—N相对应。这两个特征峰在吸附后,成分有了明显的变化,表明吸附过程中N中的孤对电子与Ni2+或Co2+结合,即—NH2和—NH与Ni2+或Co2+进行结合。而且成型壳聚糖中与C—OH和结合水相对应的532.5 eV的特征峰强度有所增加而533.4 eV的有所降低,表明成型壳聚糖中的O与Ni2+或Co2+进行了反应[38]。
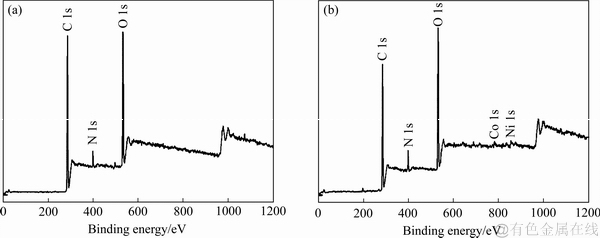
图8 CTS吸附Ni2+和Co2+前后的XPS宽扫描图
Fig. 8 XPS wide scanning maps of CTS before(a) and after(b) adsorption
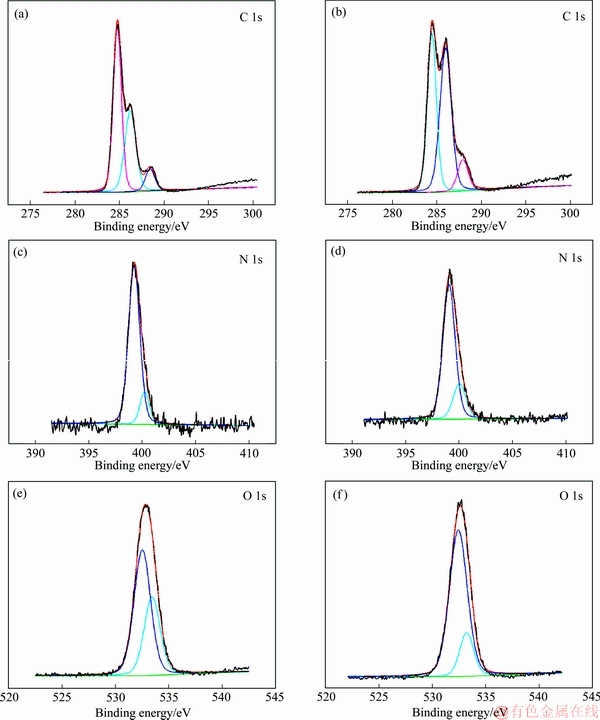
图9 成型壳聚糖吸附前后C 1s、O 1s和N 1s的能谱
Fig. 9 Energy spectra of C 1s, O 1s and N 1s of CTS before((a), (c), (e)) and after((b), (d), (f)) adsorption
表5 成型壳聚糖吸附前后主光谱的结合能和摩尔分数
Table 5 Assignments of main spectral bands based on their binding energies (EB) and mole fraction (x) for chitosan before and after adsorption
3 结论
1) 利用NaOH与壳聚糖的醋酸溶液可制备出多层成型壳聚糖固体,粒度约为60 μm,且成型前后的官能团结构未发生变化。
2) 通过对成型壳聚糖在Ni-Co溶液中的吸附行为探索,发现溶液pH、Ni2+浓度和温度升高有利于成型壳聚糖对Ni2+的吸附,而降低对Co2+的吸附;在Co2+浓度升高情况下,成型壳聚糖对Co2+吸附量上升,而对Ni2+的吸附量下降;增加成型壳聚糖加入量不利于其对两种离子的吸附。
3) 成型壳聚糖对Ni2+和Co2+的吸附符合拟二级动力学方程和Freundlich方程,且通过焓变计算发现成型壳聚糖对Ni2+的吸附焓变为正值,而对Co2+的吸附焓变为负值,表明成型壳聚糖对Ni2+、Co2+的吸附为多层吸附,而且成型壳聚糖对Ni2+的吸附为吸热反应,对Co2+的吸附为放热反应。
4) 通过采用XPS手段探究成型壳聚糖吸附Ni2+、Co2+的选择性机理,发现成型壳聚糖中的—NH2和—OH含量发生变化,表明吸附反应是成型壳聚糖中的—NH2和—OH和Ni2+、Co2+结合的过程。
5) 最后将成型壳聚糖用于萃取后Co2+溶液中Ni2+的深度除杂,发现高钴低镍溶液(ρ(Co2+)=4408 mg/L,ρ(Ni2+)=73 mg/L)中在损失8.8%Co2+的情况下,可降低58.9%的Ni2+,即在高钴低镍溶液中成型壳聚糖可对Ni2+进行吸附除杂。
REFERENCES
[1] 马立文, 聂祚仁, 席晓丽, 韩新罡. Co-Ni-Fe-Mn 配合-沉淀体系的平衡热力学[J]. 中国有色金属学报, 2013(2): 516-527.
MA Li-wen, NIE Zuo-ren, XI Xiao-li , HAN Xin-gang. Thermodynamic equilibrium in Co-Ni-Fe-Mn complexation- precipitation system[J]. The Chinese Journal of Nonferrous Metals, 2013(2): 516-527.
[2] 韩新罡, 席晓丽, 马立文, 聂祚仁, 周志理. 配合-沉淀体系中铁与钴、镍的分离性能[J]. 中国有色金属学报, 2013, 23(11): 3218-3224.
HAN Xi-gang, XI Xiao-li, MA Li-wen, NIE Zuo-ren, ZHOU Zhi-li. Separation properties of ion to cobalt and nickel in complexation-precipitation system[J]. The Chinese Journal of Nonferrous Metals, 2013, 23(11): 3218-3224.
[3] 王明欣, 孙润超, 张建国, 韩花琪, 程 丽. 化学沉淀中和法处理人造金刚石含镍酸性废水[J]. 广州化工, 2013, 41(13): 192-193.
WANG Ming-xin, SUN Run-chao, ZHANG Jiang-guo, HAN Hua-qi, CHENG Li. Chemical precipitation neutralization treatment of artificial diamond acid wastewater containing nickel[J]. Guangzhou Chemical Industry, 2013, 41(13): 192-193.
[4] SHIH Y J, LIN C P, HUANG Y H. Application of Fered-Fenton and chemical precipitation process for the treatment of electroless nickel plating wastewater[J]. Separation & Purification Technology, 2013, 104(4): 100-105.
[5] LIU Y, LEE M. Separation of cobalt and nickel from aqueous solution[J]. Journal of Molecular & Cellular Cardiology, 2013, 22(1): 415-437.
[6] NADIMI H, FATMEHSARI D H, FIROOZI S. Separation of Ni and Co by D2EHPA in the presence of citrate ion[J]. Metallurgical & Materials Transactions B, 2017, 48(5): 2751-2758.
[7] 贾战江. 连续净化黑镍除钴浅谈[J]. 现代经济信息, 2012(10): 255.
JIA Zhan-jiang. Continuous purification of cobalt in black nickel[J]. Modern Economical Information, 2012(10): 255.
[8] 徐彦宾, 谢燕婷, 闫 兰, 杨汝栋. 硫化物沉淀法从氧化镍矿酸浸液中富集有价金属[J]. 有色金属(冶炼部分), 2006(3): 8-10.
XU Yan-bin, XIE Yan-ting, YAN Lan, YANG Ru-dong. Enriching Ni, Cu, Co from acid-leaching solution of NiO ore with sulfide precipitation process[J]. Nonferrous Metals (Extractive Metallurgy), 2006(3): 8-10.
[9] WANG R C, LIN Y C, WU S H. A novel recovery process of metal values from the cathode active materials of the lithium-ion secondary batteries[J]. Hydrometallurgy, 2009, 99(3/4): 194-201.
[10] 李 凡, 朱 军, 白苗苗, 赵兴红, 赵 奇, 朱明明, 余 娟. 用溶剂萃取法从硫酸镍溶液中去除钙、铁[J]. 湿法冶金, 2016, 35(2): 132-135.
LI Fan, ZHU Jun, BAI Miao-miao, ZHAO Xing-hong, ZHAO Qi, ZHU Ming-ming, YU Juan. Removal of calcium and iron from nickel sulfate solution by solvent extraction[J]. Hydrometallurgy of China, 2016, 35(2): 132-135.
[11] 胡 威, 黄兴远, 李小理. 低浓度钴溶液除铁、钙、镁和P204深度除杂工艺研究[J]. 稀有金属与硬质合金, 2011, 39(3): 10-12.
HU Wei, HUANG Xing-yuan, LI Xiao-li. Process Study of Fe, Ca and Mg removal and deep impurity removal with P204 from low-concentration cobalt solution[J]. Rare Metals and Cemented Carbides, 2011, 39(3): 10-12.
[12] KANG J G, SENANAYAKE G, SOHN J S, SHIN S M. Recovery of cobalt sulfate from spent lithium ion batteries by reductive leaching and solvent extraction with Cyanex 272[J]. Hydrometallurgy, 2010, 100(3/4): 168-171.
[13] MISHRA R K, ROUT P C, SARANGI K, NATHSARMA K C. Solvent extraction of zinc, manganese, cobalt and nickel from nickel laterite bacterial leach liquor using sodium salts of TOPS-99 and Cyanex 272[J]. Transactions of Nonferrous Metals Society of China, 2016, 26(1): 301-309.
[14] 邬建辉, 董 波, 张献鹏, 叶逢春, 王红军, 纪宏巍, 郭方颖, 邱士伟, 刘志东. 用P507从硫酸镍溶液中萃取分离铜、锌、钴[J]. 有色金属科学与工程, 2018(2): 19-24.
WU Jiang-hui, DONG Bo, ZHANG Xian-peng, YE Feng-chun, WANG Hong-jun, JI Hong-wei, GUO Fang-ying, QIU Shi-wei, LIU Zhi-jun. Solvent extraction of Cu, Zn, Co from nickel sulphate solution applying P507[J]. Nonferrous Metals Science and Engineering, 2018(2): 19-24
[15] VIROLAINEN S, FINI M F, LAITINEN A, SAINIO T. Solvent extraction fractionation of Li-ion battery leachate containing Li, Ni, and Co[J]. Separation and Purification Technology, 2017, 179: 274-282.
[16] 刘美荣, 周桂英, 温建康. P507与Cyanex272协同萃取分离溶液中钴镍离子[J]. 过程工程学报, 2012, 12(3): 415-419.
LIU Mei-rong, ZHOU Gui-ying, WEN Jiang-kang. Separation of divalent cobalt and nickel ions using a synergistic solvent extraction system with P507 and Cyanex272[J]. The Chinese Journal of Process Engineering, 2012, 12(3): 415-419.
[17] SHEN Y, XUE W Y, NIU W Y. Recovery of Co(Ⅱ) and Ni(Ⅱ) from hydrochloric acid solution of alloy scrap[J]. Transactions of Nonferrous Metals Society of China, 2008, 18(5): 1262-1268.
[18] ONGHENA B, VALGAEREN S, HOOGERSTRAETE T V, BINNEMANS K. Cobalt(Ⅱ)/nickel(Ⅱ) separation from sulfate media by solvent extraction with an undiluted quaternary phosphonium ionic liquid[J]. RSC Advances, 2017, 7(57): 35992-35999.
[19] SUN X Q, JI Y, ZHANG L N, CHEN J, LI D Q. Separation of cobalt and nickel using inner synergistic extraction from bifunctional ionic liquid extractant (Bif-ILE)[J]. Journal of Hazardous Materials, 2010, 182: 447-452.
[20] SRIDHAR V, VERMA J K. Extraction of copper, nickel and cobalt from the leach liquor of manganese-bearing sea nodules using LIX 984N and ACORGA M5640[J]. Minerals Engineering, 2011, 24(8): 959-962.
[21] 陈 松, 王瑞忠, 王文祥, 李启厚, 刘志宏, 张多默. N235萃取净化氯化镍溶液的有机相组成及其工艺的研究[J]. 矿冶工程, 2001, 21(1): 56-58.
CHEN Song, WANG Rui-zhong WANG Wen-xiang, LI Qi-hou, LIU Zhi-hong, ZHANG Duo-mo. Study on organic phase composition and extractive process for purifying nickel chloride solution with N235[J]. Mining and Metallurgical Engineering, 2001, 21(1): 56-58.
[22] 李全民, 申义阳, 张青芬, 刘 奇. 硫酸铵-丁二酮肟-乙醇体系萃取分离钴的研究[J]. 冶金分析, 1999, 19(4): 8-10.
LI Q M, SHEN Y Y, ZHANG Q F, LIU Qi. Study on extraction of cobalt with (NH4)2SO4-biacetyl dioxime- ethanol system[J]. Metallurgical Analysis, 1999, 19(4): 8-10.
[23] YANG Y, XU S M, XIE M, HE Y H, HUANG G Y, YANG Y C. Growth mechanisms for spherical mixed hydroxide agglomerates prepared by co-precipitation method: A case of Ni1/3Co1/3Mn1/3(OH)2[J]. Journal of Alloys and Compounds, 2015, 619: 846-853.
[24] VIJAYALAKSHMI K, GOMATHI T, LATHA S, HAJEETH T, SUDHA P N. Removal of copper(Ⅱ) from aqueous solution using nanochitosan/sodium alginate/microcrystalline cellulose beads[J]. International Journal of Biological Macromolecules, 2016, 82(7): 440-452.
[25] ZENG M, ZHANG T A, Lü G Z, DOU Z H, LIU Y, ZHANG Y. Adsorption of Au(Ⅲ) ions on xanthated crosslinked chitosan resin in hydrochloric acid medium[J]. Rare Metals, 2014: 1-6.
[26] KYZAS G Z, SIAFAKA P I, PAVLIDOU E G, CHRISSAFIS K J, BIKIARIS D N. Synthesis and adsorption application of succinyl-grafted chitosan for the simultaneous removal of zinc and cationic dye from binary hazardous mixtures[J]. Chemical Engineering Journal, 2015, 259: 438-448.
[27] DEMARCHI C A, DEBRASSI A, MAGRO J D, NEDELKO N, SLAWSKA-WANIEWSKA A, DLUZEWSKI P, GRENECHE J M, RODRIGUES C A. Adsorption of Cr(Ⅵ) on crosslinked chitosan–Fe(Ⅲ) complex in fixed-bed systems[J]. Journal of Water Process Engineering, 2015, 7: 141-152.
[28] SOBHANARDAKANI S, ZANDIPAK R, JAVANSHIR- KHOEI A, HOSSEINI S M, MOSLEMI M, DELFIEH P. Removal of Hg(Ⅱ) and Cd(Ⅱ) ions from aqueous solution using chitosan: Kinetics and equilibrium studies[J]. Iranian Journal of Health Sciences, 2015, 3(2): 21-30.
[29] LIAO B, SUN W, GUO N, DING S L, SU S J. Equilibriums and kinetics studies for adsorption of Ni(Ⅱ) ion on chitosan and its triethylenetetramine derivative[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2016, 501: 32-41.
[30] REN Y, ABBOOD H A, HE F B, PENG H, HUANG K X. Magnetic EDTA-modified chitosan/SiO2/Fe3O4 adsorbent: preparation, characterization, and application in heavy metal adsorption[J]. Chemical Engineering Journal, 2013, 226: 300-311.
[31] HO Y S, MCKAY G. A comparison of chemisorption kinetic models applied to pollutant removal on various sorbents[J]. Trans IChemE B, 1998, 76(4): 332-340.
[32] SHU J X, WANG Z H, HUANG Y J, HUANG N, REN C G, ZHANG W. Adsorption removal of Congo red from aqueous solution by polyhedral Cu2O nanoparticles: Kinetics, isotherms, thermodynamics and mechanism analysis[J]. Journal of Alloys and Compounds, 2015, 633(5): 338-346.
[33] ZHOU L M, WANG Y P, LIU Z R, HUANG Q W. Characteristics of equilibrium, kinetics studies for adsorption of Hg(Ⅱ), Cu(Ⅱ), and Ni(Ⅱ) ions by thiourea-modified magnetic chitosan microspheres[J]. Journal of Hazardous Materials, 2009, 161(2/3): 995-1002.
[34] OLADOJA N A, ABOLUWOYE C O, OLOLADE I A, ADEBAYO O L, OLASENI S E, ADELAGUN R O A. Intercalation of gastropod shell derived calcium oxide in clay and application in phosphate removal from aqua medium[J]. Industrial & Engineering Chemistry Research, 2012, 51(45): 14637-14645.
[35] LIU Y, BAI Q, LOU S, DI D L, LI J T, GUO M. Adsorption characteristics of (-)-epigallocatechin gallate and caffeine in the extract of waste tea on macroporous adsorption resins functionalized with chloromethyl, amino, and phenylamino groups[J]. Journal of Agricultural and Food Chemistry, 2012, 60(6): 1555-1566.
[36] KIM Y H, KIM C M, CHOI I H, RENGARAJ S, YI J H. Arsenic removal using mesoporous alumina prepared via a templating method[J]. Environmental Science & Technology, 2004, 38(3): 924-931.
[37] 李凯斌,沈一丁,费贵强,李仲谨. 阴离子β-环糊精/Fe3O4磁性微球对Cu2+的吸附[J]. 化工学报, 2014, 65(6): 2148-2155.
LI Kai-bin, SHEN Yi-ding, FEI Gui-qiang, LI Zhong-jin. Adsorption of Cu2+ by anionic β-cyclodextrin/Fe3O4 magnetic microspheres[J]. CIESC Journal, 2014, 65(6): 2148-2155.
[38] LIAO B, GUO N, SU S J, DING S L, SUN W Y. Efficient separation and high selectivity for cobalt and nickel from manganese solution by a chitosan derivative: Competitive behavior and interaction mechanisms[J]. Industrial & Engineering Chemistry Research, 2017, 56: 3418-3428.
Selective adsorption separation of Ni and Co by modified chitosan
ZOU Xiao-ping1, 2, WANG Hai-bei1, 2, WANG Hong-yan3, 4, HUANG Kai4, LI Ping3
(1. Beijing General Research Institute of Mining & Metallurgy, Beijing 100160, China;
2. School of Metallurgy, Northeastern University, Shenyang 110006, China;
3. National Engineering Laboratory for Hydrometallurgical Cleaner Production Technology, Institute of Process Engineering, Chinese Academy of Sciences, Beijing 100190, China;
4. School of Metallurgical and Ecological Engineering, University of Science and Technology Beijing, Beijing 100083, China)
Abstract: Ni and Co are difficult from separation due to their similar physicochemical properties. Based on the difference in complexation ability between ammonia and Ni/Co, selective separation of Ni2+ and Co2+ was conducted by using modified chitosan as adsorbent with amino group. The results show that the modified chitosan with the size of 60 μm can be prepared by mixing chitosan-acetic acid solution with NaOH. The adsorption behavior of Ni2+ and Co2+ onto modified chitosan, including adsorption isotherm and kinetics, is fitted well by Freundlich model and pseudo-second- order model, respectively. Adsorption enthalpy results indicate that the Ni2+ uptake onto modified chitosan is endothermic while the Co2+ uptake is exothermic. The adsorption mechanism shows that the Ni2+ and Co2+ are chelated with amino and hydroxyl groups of modified chitosan. In the specific application, Ni2+ content of high concentrated Co2+ solutions decreases from 73×10-6 to 30×10-6, realizing the selective separation of Ni.
Key words: nickel and cobalt; adsorption; selective separation; chitosan; modification
Foundation item: Projects(51574212, U1403195) supported by the National Natural Science Foundation of China
Received date: 2018-07-03; Accepted date: 2018-12-18
Corresponding author: LI Ping; Tel: +86-10-82544856; E-mail: lipinggnipil@home.ipe.ac.cn
(编辑 何学锋)
基金项目:国家自然科学基金资助项目(51574212,U1403195)
收稿日期:2018-07-03;修订日期:2018-12-18
通信作者:李 平,副研究员,博士;电话:010-82544856;E-mail:lipinggnipil@ipe.ac.cn
摘 要:本文以成型壳聚糖为吸附剂进行溶液中Ni-Co的分离,通过Ni2+和Co2+与壳聚糖上氨基的络合能力差异达到Ni2+、Co2+分离的目的。结果表明:利用NaOH与壳聚糖醋酸溶液可制备出粒度约为60 μm的层状成型壳聚糖;成型壳聚糖对Ni2+、Co2+的吸附符合拟二级动力学方程和Freundlich方程;经吸附焓变计算发现成型壳聚糖对Ni2+的吸附为吸热反应,而对Co2+的吸附为放热反应;吸附机理表明,壳聚糖上的氨基和羟基与Ni2+、Co2+发生结合反应。以萃取分离后得到的高Co2+低Ni2+溶液为原料,成型壳聚糖吸附后溶液中Ni2+含量由73×10-6降低至30×10-6,实现杂质的深度脱除。
[1] 马立文, 聂祚仁, 席晓丽, 韩新罡. Co-Ni-Fe-Mn 配合-沉淀体系的平衡热力学[J]. 中国有色金属学报, 2013(2): 516-527.
[2] 韩新罡, 席晓丽, 马立文, 聂祚仁, 周志理. 配合-沉淀体系中铁与钴、镍的分离性能[J]. 中国有色金属学报, 2013, 23(11): 3218-3224.
[3] 王明欣, 孙润超, 张建国, 韩花琪, 程 丽. 化学沉淀中和法处理人造金刚石含镍酸性废水[J]. 广州化工, 2013, 41(13): 192-193.
[7] 贾战江. 连续净化黑镍除钴浅谈[J]. 现代经济信息, 2012(10): 255.
[8] 徐彦宾, 谢燕婷, 闫 兰, 杨汝栋. 硫化物沉淀法从氧化镍矿酸浸液中富集有价金属[J]. 有色金属(冶炼部分), 2006(3): 8-10.
[10] 李 凡, 朱 军, 白苗苗, 赵兴红, 赵 奇, 朱明明, 余 娟. 用溶剂萃取法从硫酸镍溶液中去除钙、铁[J]. 湿法冶金, 2016, 35(2): 132-135.
[11] 胡 威, 黄兴远, 李小理. 低浓度钴溶液除铁、钙、镁和P204深度除杂工艺研究[J]. 稀有金属与硬质合金, 2011, 39(3): 10-12.
[16] 刘美荣, 周桂英, 温建康. P507与Cyanex272协同萃取分离溶液中钴镍离子[J]. 过程工程学报, 2012, 12(3): 415-419.
[21] 陈 松, 王瑞忠, 王文祥, 李启厚, 刘志宏, 张多默. N235萃取净化氯化镍溶液的有机相组成及其工艺的研究[J]. 矿冶工程, 2001, 21(1): 56-58.
[22] 李全民, 申义阳, 张青芬, 刘 奇. 硫酸铵-丁二酮肟-乙醇体系萃取分离钴的研究[J]. 冶金分析, 1999, 19(4): 8-10.
[37] 李凯斌,沈一丁,费贵强,李仲谨. 阴离子β-环糊精/Fe3O4磁性微球对Cu2+的吸附[J]. 化工学报, 2014, 65(6): 2148-2155.


