Article ID: 1003-6326(2005)06-1275-05
Interpretation on Sebisty effect of hot-dip galvanized steels
CHE Chun-shan(车淳山), LU Jin-tang(卢锦堂), KONG Gang(孔 纲)
(College of Materials Science and Engineering,
South China University of Technology, Guangzhou 510640, China)
Abstract:
Sebisty effect describes the unusual fact that the thickness of the hot-dip galvanizing coating on the steel containing 0.12%-0.25% silicon decreases with increasing temperature of zinc bath. The microstructures of hot-dip galvanized coatings on silicon-containing steels (0.14%Si) immersed in zinc bath at 723K and 753K were analyzed. It is found that the thickness of η and ζ layer decreases with the increase of temperature of zinc bath and Г layer changes from discontinuous layer (at 723K) to relatively continuous layer (at 753K). The improvement of the fluidity of zinc bath due to the rising temperature of zinc bath makes η layer thinner. Moreover, the existence of relatively continuous Г layer and the acceleration of the dissolution of ζ layer to zinc bath co-lead to the decrease of the thickness of ζ layer with increasing temperature.
Key words:
zinc coatings; Sebisty effect; intermetallics; galvanized steel CLC;
number: TG74.443 Document code: A
1 INTRODUCTION
The quality of hot-dip galvanized products is greatly influenced by many factors such as the constituents in steels[1, 2], the elements in zinc bath[3-5], and hot-dip galvanizing temperature. The constituents in steels that have the greatest influence on the galvanized coatings are silicon, which is frequently added to steel as a deoxidant during its production. Sandelin[6] firstly reported that it was the silicon in steels that caused excessively galvanized coatings on steel articles. For a typical low-carbon steel article containing little silicon, a continuous and compact zinc protective coating with various intermetallic layers composed of Γ, δ, ζ and η is developed on the surface of a steel article, in accordance with a Fe-Zn binary phase diagram. The presence of silicon at certain levels (in the vicinity of 0.1% and above 0.3%(mass fraction)) in steels, known as reactive steels, can result in the rapid growth of the alloy layer, producing a coating of excessive thickness, having gray appearance and poor adherence[7-9]. However, it is surprising that the steel articles with silicon content between 0.12% and 0.25%(mass fraction) produce continuous and compact coatings whose thickness decreases with increasing temperature of zinc bath. The unusual phenomena is first observed by Sebisty, called Sebisty effect. In galvanizing industry, silicon content in a large number of steel articles locates in the range of 0.1%-0.25%. Until now, the Sebisty effect has not been insufficient of experimental proof[10]. In this paper, by investigating the microstructures of hot-dip galvanized coatings on silicon-containing steels (0.14%Si) immersed in zinc bath at 723K and 753K, the interpretation of Sebisty effect was proposed.
2 EXPERIMENTAL
The 55mm×40mm×3mm samples were hot-rolled steel sheets. Their chemical compositions are listed in Table 1.
Table 1 Chemical compositions of samples (mass fraction, %)

Before being dipped in the zinc bath, the samples were prepared in a classical galvanizing treatment cycle. They were ground and then successively cleaned in an alkaline solution, pickled in an acid solution, fluxed in an ammonium chloride and zinc chloride solution and dried at 393K. Fluxing enables steel samples to be protected before galvanizing and promotes the attack of the substrate by zinc during the first seconds of reaction. The samples were then dipped in pure zinc bath at (723±2)K and (753±2)K for immersion time varying from 3min to 10min in a laboratory simulator. After galvanizing treatment, they were quenched quickly in water. The cross-sections of the samples were analyzed by scanning electron microscopy (SEM: LEO 1530VP) coupled with an energy-dispersive spectroscopy system (EDS: INCA 300) after they were polished and etched with chromic acid liquor.
3 RESULTS AND ANALYSES
3.1 Microstructural change of coatings
When galvanized at 723K and 753K for 6min, the samples have similar coatings with compact and continuous δ, ζ and η layer, just like those of low-silicon non-reactive steels, as shown in Figs.1(a) and (b). At 723K, the coating is about 157μm in thickness including δ(12μm), ζ(103μm) and η(42μm) layer. At 753K, the coating thickness decreases by 36μm, which is 121μm including δ(13μm), ζ(83μm) and η(25μm), as illustrated in Fig.2.
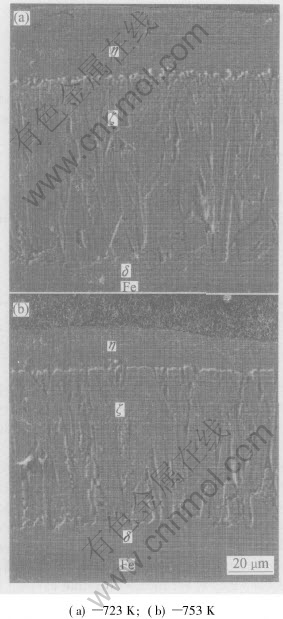
Fig.1 Microstructures of galvanized coating on 0.14% Si steel at different temperatures for 6min
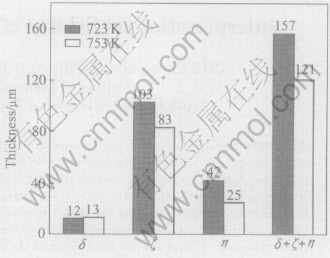
Fig.2 Thickness of galvanized coating on 0.14%Si steel at different temperatures for 6min
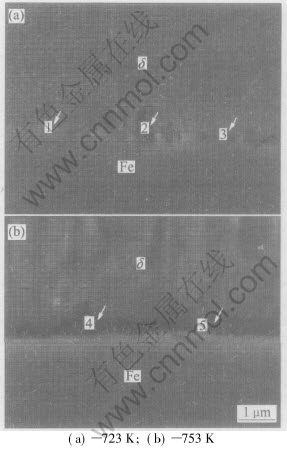
Fig.3 Microstructures of substrate/coating interface of galvanized coating on 0.14%Si steel at different temperatures for 6min
3.2 Change of steel substrate/coating interface
The microstructure of the substrate/coating interface was observed using SEM under high magnification, as shown in Fig.3. It is clear that Г is discontinuous at 723K(Fig.3(a)), while Г becomes a relatively continuous layer with a thickness of less than 0.5μm at 753K, as shown in Fig.3(b). EDS results show that the point 1 marked in Fig.3 contains about 11%Fe(mass fraction), identified as δ phase (FeZn10, 7.0%-11.5%), while the points 2, 3, 4 and 5 contain about 25%-30% Fe, identified as Γ phase(Fe3Zn10, 23%-27.7%).
4 DISCUSSION
By comparing the microstructure of the galvanized coating at 723K with that at 753K, it is found that there are three changes in the coatings with increasing temperature of zinc bath. The first one is the decrease of the thickness of the η layer, the second one is the change of the Г layer from discontinuous to relatively continuous, the last one is the decrease of the thickness of the ζ layer.
4.1 Change of η layer
Above 693K, zinc is in a liquid state. The viscosity μ of the zinc is dependent upon temperature, and is determined by the equation of μ=μ0exp(E/RT)[11]. Where μ0 is the coefficient of viscosity at melting temperature, 0.4131×10-3N·s/m2[11], E is the activation energy, 12700J/mol[11], R is the universal gas constant, 8.314J/(mol·K). At 723K, the viscosity of zinc is 3.42×10-3N·s/m2. When the temperature of the zinc bath is increased to 753K, the viscosity of zinc is decreased to 3.14×10-3N·s/m2. The decrease of the viscosity of zinc with rising temperature of the zinc bath improves the fluidity of zinc bath, which is in favor to the liquid zinc flowing back to zinc bath on the surface of the samples when they are drawn away from zinc bath, resulting in the η layer thinner.
4.2 Change of Г layer
Temperature is one of the key factors which influence the Fe-Zn reaction. The higher the temperature is, the easier the Fe-Zn reaction takes place. The growth rate of Fe-Zn compounds is usually quickened with the increase of temperature. However, it is also possible that the growth rate of Fe-Zn compounds is lowered if phase transformation occurs during Fe-Zn reaction.
Kozdrs et al[12] carried on the research of the galvanizing steels with less than 0.2% silicon. It is found that Г layer plays a major role in the growth of galvanized coatings. The modification of Г layer occurs concurrently with bearded structure formation of galvanized coatings. He proposed that silicon destroying compact and continuous Г layer leads to the formation of abnormal coatings.
Because of the absence of Fe-Zn-Si ternary phase diagram at the temperature higher than 723K, it is uncertain that how temperature affects the Fe-Zn-Si phases. With regard to Sebisty steels, the substrate/coating interface is a discontinuous Г layer at low temperature(e.g. 723K) and the Г layer becomes relatively continuous at high temperature(e.g. 753K). The fact shows that Г layer containing certain silicon can keep more stable at high temperature. Г layer is the most iron-rich and compact Fe-Zn compound and its stability can control the growth of galvanized coatings, which delays the growth of the Fe-Zn compound layer.
4.3 Change of ζ layer
During galvanizing, the growth of ζ layer accompanies with ζ dissolution to zinc bath. The schematic illustration of the ζ layer growth is shown in Fig.4.
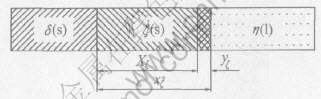
Fig.4 Schematic illustration of ζ layer growth
The growth rate of ζ layer is given by[13]
dXζ/dt=dxζ/dt-dyζ/dt(1)
where dXζ/dt is the growth rate of ζ layer, dxζ/dt is the growth rate of ζ layer without dissolution, abiding by Fick diffusion law, and dyζ/dt is the dissolving rate of ζ layer, abiding by Nernst-Bruner equation.
According to Fick law:
dxζ/dt=Kζ, 1/xζ(2)
where Kζ, 1 is the growth coefficient.
According to Nernst-Bruner equation:
dCζ/dt=Kζ, 2(S/V)(CSFe-Cζ)(3)
where dCζ/dt is the change rate of iron concentration in zinc bath, Kζ, 2 is the dissolution coefficient, S is the surface area of samples, and V is the volume of zinc bath and CSFe is the iron saturated concentration in zinc bath.
Cζ=ρζγζSyζ/V(4)
where ρζ is the density of ζ layer, γζ is the average Fe concentration in ζ layer and yζ is the average thickness of the dissolved ζ layer.
V is approximately considered a constant as it hardly changes with the change of Fe solubility in zinc bath.
Substituting Eqn.(4) to (3) and getting
dyζ/dt=-ayζ+b(5)
where a=Kζ, 2S/V and b=Kζ, 2CSζ/ρζγζ.
Combining Eqns.(1), (2) and (5), the growth rate of ζ layer is denoted by
dXζ/dt=Kζ, 1/Xζ+ayζ-b(6)
During galvanizing, VS, so a→0, Eqn.(6 ) is simplified by
dXζ/dt=Kζ, 1/Xζ-b(7)
Integrating Eqn.(7) and substituting the initial conditions t=0, Xζ=0, it is obtained that
-(Kζ, 1/b2)ln(1-bX/Kζ, 1)-X/b=t(8)
Expanding Eqn.(8) in series and taking the first three items, it is given by
X2ζ/(2Kζ, 1)+(1/3)(bX3ζ/K2ζ, 1)=t(9)
Simplifying Eqn.(9) and getting
FX2ζ+GX3ζ=t(10)
Substituting the test data to Eqn.(10) and simulating them according to the method of least squares, it is obtained that
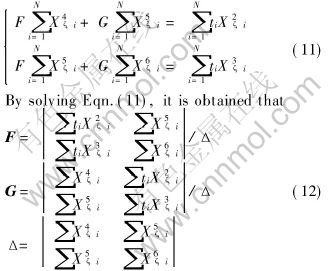
As CSFe, ρζ and γζ are the known parameters, Kζ, 1 and Kζ, 2 are acquired by Eqns.(9) and (12).
Integrating Eqn.(2) and substituting the initial conditions t=0, xζ=0, it is obtained that
![]()
Integrating Eqn.(5) and substituting the initial conditions t=0, yζ=0, it is obtained that
![]()
Integrating Eqn.(1) and substituting the initial conditions t=0, Xζ=0, Eqns.(13) and (14) , the thickness of ζ layer is obtained by
![]()
The available parameters are listed in Table 2.
Table 2 Known physical parameters
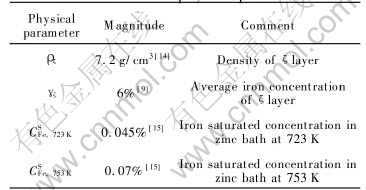
Fig.5 shows the thickness of ζ layer of the galvanized coating on 0.14%Si steel at 723K and 753K as a function of immersion time. It is clear that the thickness of ζ layer increases with increasing immersion time whereas it decreases with increasing temperature of zinc bath. By substituting the values (Xζ, i) to Eqn.(12), the values of F, G, Kζ,1 and Kζ, 2 are acquired, as shown in Table 3. Kζ, 1 is the growth coefficient of ζ layer and its value reflects the growth controlled by the diffusion mechanism. It is shown from Table 3 that Kζ, 1 decreases from 13.05 to 10.74 with the increase of galvanizing temperature from 723K to 753K. It is inferred that the growth of ζ layer controlled by the diffusion mechanism is declined, which may be due to the change of Г layer from discontinuous to relatively continuous, delaying the Fe-Zn diffusion. Kζ, 2 is the dissolution coefficient and its value reflects the dissolution of ζ layer to zinc bath. It is also seen from Table 3 that Kζ, 2 increases from 6.68 to 22.43 with the galvanizing temperature from 723K up to 753K. It is inferred that the dissolution of ζ layer to zinc bath accelerates with rising temperature. By combining Eqn.(15), it is found that the value of Xζ decreases, that is, the thickness of ζ layer decreases, if the value of Kζ, 1 decreases and the value of Kζ, 2 increases.
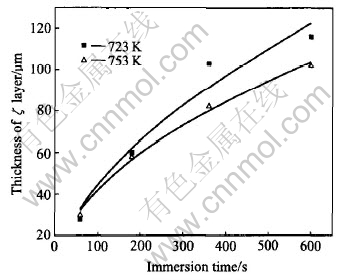
Fig.5 Thickness of ζ layer of galvanized coating on 0.14%Si steel at 723K and 753K as function of immersion time
Table 3 Values of F, G, Kζ, 1 and Kζ, 2 of ζ layer of galvanized coating on 0.14%Si steel at 723K and 753K

5 CONCLUSIONS
1) The thickness of galvanized coatings on 0.14%Si steels decreases with increasing temperature at 723-753K.
2) There are three changes in galvanized coatings with increasing temperature: the decrease of the thickness of ζ and η layer and the change of Г layer from discontinuous to relatively continuous.
3) The improvement of fluidity of zinc bath due to the rising temperature of zinc bath makes η layer thinner.
4) The existence of relatively continuous Г layer and the acceleration of the dissolution of ζ layer to zinc bath co-lead to the decrease of the thickness of ζ layer with increasing temperature.
REFERENCES
[1]Mackowiak J, Short N R. Metallurgy of galvanized coatings [J]. Inter Metals Review, 1979(1): 1-16.
[2]KONG Gang, LU Jin-tang, CHEN Jin-hong, et al. Review on effect of steel composition on batch hot dip galvanizing [J]. Corros Sci Protec Tech, 2004, 16(3): 162-165.
[3]KONG Gang, LU Jin-tang, CHEN Jin-hong, et al. Effects of elements in zinc bath on batch hot dip galvanizing [J]. Surf Tech, 2003, 32(4): 7-10.
[4]Haratoshi K, Shuji H. Effect of alloy component present in prime westen grade zinc bath [A]. Proc 4th Asian-Pacifeic General Galva Conf [C]. Kuala Lumpur: MGA, 1999. Session 6. 233-236.
[5]LU Jin-tang, CHEN Jin-hong, XU Qiao-yu, et al. Influence of adding Ni in zinc bath on the microstructure of hot dip galvanized coating [J]. The Chinese Journal of Nonferrous Metals, 1996, 6(4): 87-90.(in Chinese)
[6]Sandlein R W. Galvanizing characteristics of different types of steel [J]. Wire and Wire Products, 1941, 16: 28-35.
[7]Lynch R F. Hot-dip galvanizing alloys [J]. JOM, 1987, 7: 39-41.
[8]Chen Z W, Kennon N F, See J B, et al. Technigalva and other developments in batch hot-dip galvanizing [J]. JOM, 1992, 44(1): 22-26.
[9]Marder A R. The metallurgy of zinc-coated steel [J]. Prog Mater Sci, 2000, 45: 191-271.
[10]Schulz W D, Schubert P, Thiele M. An alternative approach to explaining the effect on the galvanizing reaction [A]. Proc 20th Inter Galva Conf [C]. Amsterdam: EGGA, 2003. 61-64.
[11]Dell S , Chales J, Vlot M, et al. Modelling of iron dissolution during hot dip galvanizing of strip steel [J]. Mater Sci Tech, 2004, 20(2): 251-256.
[12]Kozdras M S, Niessen P. Silicon-induced destabilization of galvanized coating in the sandelin peak region [J]. Metallography, 1989, 22: 253-267.
[13]LIU Gen-fan, LI Hua-fei, ZHENG Jia-shen, et al. Growth kinetics of intermetallic layer in 55%Al-Zn hot-dipping process with passivation method [J]. J Huazhong Univ of Sci & Tech(Nature Science Edition), 2002, 30(7): 7-10.(in Chinese)
[14]Claude H E Berlin, Renaud C H Berlin. Synthesis and crystal structure determinations in the Г and δ phase domains of the iron-zinc system: electronic and bonding analysis of Fe13Zn39 and FeZn10, a subtle deviation from the hume-rothery standard? [J]. J Solid State Chem, 2000, 151: 85-95.
[15]Tang N Y. Determination of liquid-phase boundaries in Zn-Fe-Mx systems [J]. J Phase Equilibrium, 2000, 21(1): 70-76.
(Edited by YUAN Sai-qian)
Received date: 2005-03-23; Accepted date: 2005-08-15
Correspondence: CHE Chun-shan, PhD; Tel: +86-20-85511540; E-mail: wyccs1975@163.com


