
Dynamics simulation of tertiary amines adsorbing on kaolinite (001) plane
LIU Chang-miao1, 2, FENG An-sheng1, 2, GUO Zhen-xu1, 2, CAO Xue-feng3, HU Yue-hua3
1. Zhengzhou Institute of Multipurpose Utilization of Mineral Resources, CAGS, Zhengzhou 450006, China;
2. China National Engineering Research Center for Utilization of Industrial Minerals, Zhengzhou 450006, China;
3. School of Minerals Processing and Bio-engineering, Central South University, Changsha 410083, China
Received 7 September 2010; accepted 31 December 2010
Abstract: The collecting power of tertiary amines (DRN, DEN and DPN) on kaolinite follows the order of DEN>DPN>DRN. After reacting with DRN, DEN and DPN, the surface potentials of kaolinite increase remarkably, and the recruitments caused by collectors also follow the order of DEN>DPN>DRN. The results of dynamics simulation show that the geometries of substituent groups bonding to N are deflected and twisted, and some of bond angles are changed when tertiary amines cations adsorb on kaolinite (001) surface. Based on the results of dynamics simulations and quantum chemistry calculations, the electrostatic forces between three tertiary amines cations and 4×4×3 (001) plane of kaolinite are 1.38×10-7 N (DRN12H+), 1.44×10-6 N (DEN12H+), 1.383×10-6 N (DPN12H+), respectively.
Key words: tertiary amines; kaolinite; (001) plane; dynamics simulation; electrostatic force
1 Introduction
Kaolinite, a typical clay-type gangue mineral present in many kinds of ores, is commonly hosted in the diasporic bauxite of China. Most of Chinese bauxites must be preprocessed to be utilized commercially by pre-removing the silica (kaolinite and other gangue minerals) to increase the ratio of Al2O3 to SiO2 (A/S). Hence, plenty of studies on the crystal structure, surface characteristics, and flotation reagents of kaolinite have been carried out in past few years. HU et al [1-6] and SUN et al [7-8] made detailed researches on the crystal structure and surface chemistry of kaolinite, and the results showed that kaolinite is a layer aluminosilicate mineral, and the charged surfaces mainly are (001) and (![]() ) in the pulp. The charge mechanism is mainly controlled by the ionization equilibrium of Al—OH and Si—OH on the kaolinite surface; meanwhile, particles of kaolinite always have a part of permanent negative charge for the isomorphic exchange of kaolinite surface ions [9].
) in the pulp. The charge mechanism is mainly controlled by the ionization equilibrium of Al—OH and Si—OH on the kaolinite surface; meanwhile, particles of kaolinite always have a part of permanent negative charge for the isomorphic exchange of kaolinite surface ions [9].
Making use of the reverse flotation technique, Chinese low grade bauxites can be beneficiated extensively [10-13]. Amine reagents are the traditional collectors in reverse flotation. To improve the floatability of aluminosilicates (kaolinite and other silica minerals), efforts were focused on testing various cationic amine collectors, and the floating relationship and reaction mechanism between amines and kaolinite were studied extensively up to now. LI et al [14] reported the molecular dynamics simulation about the adsorption of cationic collector (DDA) on kaolinite surface. The results showed that DDA has the adsorption priority on kaolinite (001) in the alkaline pulp, and DDA equally adsorbs on each surface of kaolinite in the acid pulp. In order to explain the floating discrepancy of DDA on kaolinite in the alkaline and acid pulp, HU et al [15] carried out the molecular dynamics simulation of DDA on kaolinite. These results showed that the self-aggregation between (![]() ) faces and the edge planes and the adsorption of DDA on the silica (001) plane make the kaolinite aggregates hydrophobic, and a good floatability is achieved in acidic solution. In alkaline solution, the kaolinite particles are dispersed, hydrophobic aggregation appears to occur between the (001) planes due to the adsorbed DDA, and thus the hydrophilic (
) faces and the edge planes and the adsorption of DDA on the silica (001) plane make the kaolinite aggregates hydrophobic, and a good floatability is achieved in acidic solution. In alkaline solution, the kaolinite particles are dispersed, hydrophobic aggregation appears to occur between the (001) planes due to the adsorbed DDA, and thus the hydrophilic (![]() ) faces are exposed and flotation does not achieved. LIU et al [16] studied the interaction mechanism between amines and kaolinite surfaces with the solution chemistry and quantum chemistry calculation. Their studies showed that the states of Si—OH, Si—O-, Al—OH and Al—O- at kaolinite surfaces definitively affect the adsorption of amines on kaolinite surface. The authors [17] employed a series of tertiary amines to float kaolinite. The flotation results showed that three collectors’ recovering abilities for kaolinite follow the order of DEN > DPN > DRN. The studies of quantum chemistry calculations indicated that the floatability of tertiary amines is decided by the substituent effects from the substitute groups bonding to N atom. In this work, the authors employed three dodecyl tertiary amines (DRN, DEN and DPN) to carry out the molecule dynamics simulations of these amines on kaolinite (001) surface. On the basis of simulations results, the authors try to find the adsorption mechanism of tertiary amines on kaolinite furthermore, and expose the micro- mechanism of adsorptions between cationic collectors and kaolinite surface.
) faces are exposed and flotation does not achieved. LIU et al [16] studied the interaction mechanism between amines and kaolinite surfaces with the solution chemistry and quantum chemistry calculation. Their studies showed that the states of Si—OH, Si—O-, Al—OH and Al—O- at kaolinite surfaces definitively affect the adsorption of amines on kaolinite surface. The authors [17] employed a series of tertiary amines to float kaolinite. The flotation results showed that three collectors’ recovering abilities for kaolinite follow the order of DEN > DPN > DRN. The studies of quantum chemistry calculations indicated that the floatability of tertiary amines is decided by the substituent effects from the substitute groups bonding to N atom. In this work, the authors employed three dodecyl tertiary amines (DRN, DEN and DPN) to carry out the molecule dynamics simulations of these amines on kaolinite (001) surface. On the basis of simulations results, the authors try to find the adsorption mechanism of tertiary amines on kaolinite furthermore, and expose the micro- mechanism of adsorptions between cationic collectors and kaolinite surface.
2 Experimental
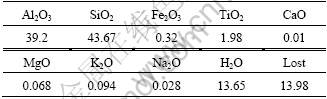
Pure minerals of kaolinite were supplied by Xiaoyi Mine of Shanxi Province, China. The sample was 95% pure based on mineralogical analysis and chemical analysis, and the chemical analysis results are given in Table 1. Fractions of <0.074 mm of kaolinite were used for micro-flotation tests.
Table 1 Chemical analyses of kaolinite (mass fraction, %)
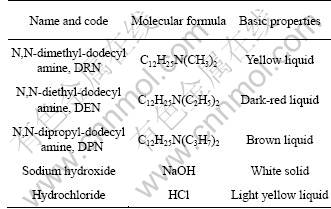
Three tertiary amines (DRN, DEN and DPN) were used as collectors. These amines were synthesized in the laboratory with the raw materials of dodecyl amine (DDA), formaldehyde (acetaldehyde and propyl- aldehyde) and formic acid, in the way of deoxidation and alkylation. And the reaction was maintained at 353.15 K for 10 h. The products were purified by the rotary vacuum evaporator several times [18]. And the purities of DRN, DEN and DPN are about 90%, 85% and 70%, respectively. Analytical grade sodium hydroxide and hydrochloric acid were used for pH control. The details of all these reagents are given in Table 2.
Table 2 Details of reagents used in flotation tests
2.3.1 Micro-flotation
Micro-flotation tests were carried out on the flotation machine of XFG type. 3 g pure mineral particles of kaolinite were placed in a plexiglass cell (40 mL), which was then filled with about 35 mL distilled water. After adding the desired amount of reagents, the suspension was agitated for 3 min, and the pH value was adjusted before a 5 min flotation period. The products and tailings were weighed separately after filtration and drying, and then the recovery was calculated.
2.3.2 Measurement of zeta potential
Pure minerals of kaolinite were ground to a 5 μm diameter, and then 0.01 mol/L suspension was prepared with distilled water. After the pH value was adjusted and the desired collector was introduced into the suspension, zeta potentials were measured using a Brookhaven Zeta Plus Zeta-potential analyzer (USA). All measurements were conducted in a 0.1 mol/L KNO3 background electrolyte solution.
2.3.3 Dynamics simulation
Based on the Compass force field, dynamics simulations were conducted in the Discover modules of Material Studio 4.0. The crystal unit-cell of kaolinite was imported to the work project. After the suitable 4×4×3 (001) plane was cleaved by the edit tools, a vacuum slab was built to accommodate the (001) plane. O atoms bonding to Si were assigned to the O_Si force field from the silicon-oxygen tetrahedron and the Si atoms were endowed with Si4_O force field from the silicon-oxygen tetrahedron. O atoms bonding to Al were selected to the Al_O force field in the Al2O3, and Al atoms were selected to the Al3_O force field. These O atoms bonding to both Si and Al were assigned to O_Si and Al_O each half. Meanwhile, the kaolinite cell including (001) plane was optimized by Compass force field to ensure the best stability and reliability. After drawing the configuration of tertiary amine cations to the work project correctly, a suitable amorphous cell was constructed to hold the tertiary amine cations. Using the tools of Build Layers, the constructed 4×4×3 (001) plane and the amorphous cell holding the cations were merged to be a whole cell. The 4×4×3 (001) plane was at the down layer with a distance of 10 nm away from the amorphous at the up layer. In order to decrease the complication degree and save the calculation time, the dynamics simulation was carried out in the ideal vacuum. The module is shown in Fig. 1. The simulation process was 300 000 steps, and the temperature was 25 °C.
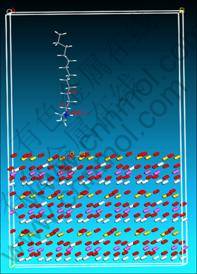
Fig. 1 Interaction module of tertiary amine cations on 4×4×3 (001) plane of kaolinite
3 Results and discussion
The recoveries of kaolinite as a function of pH value are shown in Fig. 2 using tertiary amines of DRN, DEN and DPN. In contrast with the high recoveries in acid pulp, the recoveries of kaolinite are quite low in alkaline pulp; and this may be aroused by different compositions of tertiary amines and changing states of kaolinite surface in acid and alkaline pulp [16]. The collecting powers of these three tertiary amines follow the order of DEN > DPN > DRN.
On the basis of results from JIANG et al [19], ZHAO et al [9], CAO et al [20], XIA et al [12], LIU et al [17], the interaction mechanisms between various cationic amine reagents and kaolinite surfaces are mainly physics adsorption controlled by electric-static power. In order to confirm the interaction mechanism of tertiary amine cations on kaolinite surfaces, the influences of three collectors on the zeta potentials of kaolinite were tested (Fig. 3). The results indicated that zeta potentials of kaolinite increased a lot after reacting with three tertiary amines, and the recruitment of zeta potential aroused from DEN is the biggest. According to zeta potential measurement results, the interaction level between three collectors and kaolinite surfaces follows the order of DEN > DPN > DRN, and is consistent with the flotation results.
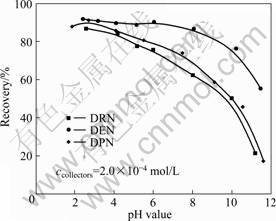
Fig. 2 Recoveries of kaolinite as function of pH value with three tertiary amines
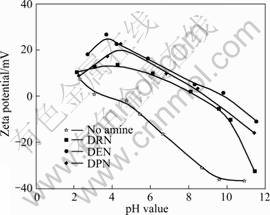
Fig. 3 Effects of tertiary amines on zeta potential of kaolinite (ccollectors = 2×10-4 mol/L)
In order to explain the interaction discrepancy of three collectors on kaolinite surface, the dynamics simulations of tertiary amine cations on kaolinite (001) plane were conducted. According to the statistical data, electro-static force between tertiary amines cations and kaolinite (001) plane was calculated to explain the floatability discrepancy of three collectors. Based on the constructed modules, the dynamics simulations of three collectors’ cations (DRN12H+, DEN12H+ and DPN12H+) and kaolinite (001) plane were carried out. The initial and final states are shown in Fig. 4 for DRN12H+, in Fig. 5 for DEN12H+, and in Fig. 6 for DPN12H+, respectively.
After tertiary amine cations steadily adsorb on kaolinite (001) plane, the shapes of substituent groups bonding to N atom in final state are deflected and twisted compared with that in initial state. The deflected and twisted level of two propyls from DPN12H+ is the highest, that of two ethyls from DEN12H+ is the next, and that of two methyls from DRN12H+ is the lowest. The underlying reason may be the steric effect aroused from substituent groups. The bigger space size causes the stronger space steric effect, and leads to the higher deflected and twisted extent while tertiary amines cations are closing to kaolinite (001) plane. Meanwhile, the deflected and twisted phenomena are as well as confirmed by transformations of some angles in head groups of collectors cations. The angles of H—N—C in DRN12H+, C—N—C in DEN12H+, and N—C—C in DPN12H+ were changed from 104°, 111° and 122° to 90°, 101° and 112°, respectively (Fig. 7).
Based on the results of Figs. 4-6, after DRN12H+, DEN12H+ and DPN12H+ adsorb stably on kaolinite (001) surface, the distances of N atom away from (001) surface decrease, and three cations are close to (001) surface much more compared with the initial situation. In order to gain the change trend of the distance of N atom away from (001) surface in the process of simulation, we made the statistic analysis of the distance of N atom away from (001) surface in the way of analysis function of MS station, and the results are shown in Fig. 8. The results indicate that the distances in the final state of N atom in three cations away from (001) surface are 2.99 ?, 2.94 ? and 3.001 ? for DRN12H+, DEN12H+ and DPN12H+ respectively. It seems that N atom in DENH+ is close to (001) surface much more than that in DRN12H+ or DPN12H+.
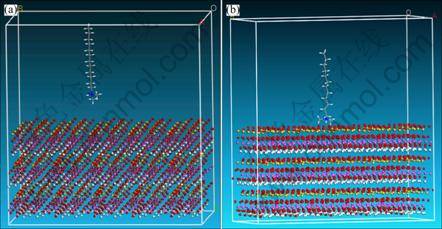
Fig. 4 Initial (a) and final (b) states of DRN12H+ on (001) plane
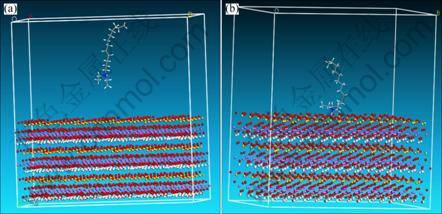
Fig. 5 Initial (a) and final (b) states of DEN12H+ on (001) plane
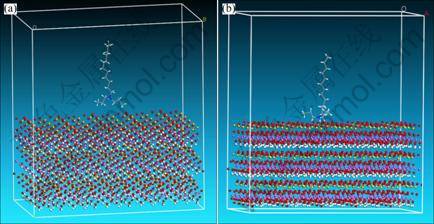
Fig. 6 Initial (a) and final (b) states of DPN12H+ on (001) plane
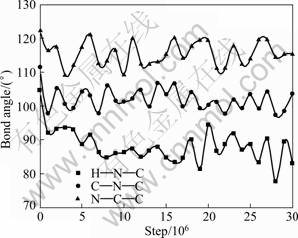
Fig. 7 Angles of H—N—C in DRN12H+, C—N—C in DEN12H+ and N—C—C in DPN12H+ as function of simulation steps
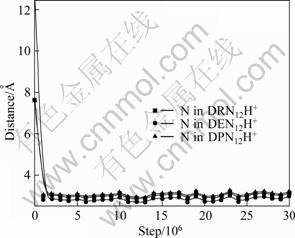
Fig. 8 Distances of N atom in DRN12H+, DEN12H+ and DPN12H+ away from (001) surface as function of simulation steps
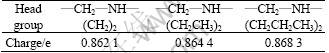
Based on the theory of Coulomb’s theory [21], the electro-static forces between tertiary amine cations and (001) plane of kaolinite are determined by their charges and distances. Without consideration of charges, the shorter distance would lead to the stronger electro-static effect (the higher attractive force). In order to calculate the electro-static force between these cations and (001) plane of kaolinite, their charges must be found. Three tertiary amines have the same hydrophobic carbon chains. According to the valence-bond and charge-equilibrium theory, almost the whole positive charges of collectors’ cations distribute in the head group (ammonium group), and only very little positive charges distribute in the hydrophobic carbon chains. With the purpose of confirming the charge distribution of the head groups, the quantum chemistry calculation on three cations (DRN12H+, DEN12H+ and DPN12H+) was carried out using the module of Dmol3 in Material Studio 4.0 (Table 3).
Table 3 Charges of head groups in three tertiary amine cations
From the dynamics simulation foundation, we know that O atoms in (001) plane of kaolinite are assigned to the Si4_O force field from the silicon-oxygen tetrahedron. Using the function of calculating charge in MS station, the unit charge of O atom in this force field is calculated to be -0.5 e. There are 125 O-atoms in a 4×4×3 (001) plane. Hence, a 4×4×3 (001) plane is charged with -62.5 e.
With the charges of head groups of tertiary amines cations, that of 4×4×3 (001) plane of kaolinite and the distances between each other, the electro-static forces of collector cations interacting with (001) plane can be gained by the Coulomb’s formula: F= Kq1q2/r2. From the results listed in Table 4, the electro-static forces between three cations and 4×4×3 (001) plane follow the order of DEN12H+> DPN12H+>DRN12H+.
Table 4 Electrostatic force between cations of tertiary amines and 4×4×3 (001) plane

For the electro-static force, three tertiary amine cations are close to (001) plane of kaolinite, and adsorb onto it finally. For the steric effects, the shapes of substituent bonding to N atom in the head groups are deflected and twisted, and some angles are changed. On the basis of calculation results, the electro-static forces between three tertiary amines cations and (001) plane are different from each other (DEN12H+>DPN12H+> DRN12H+). And this can also explain their discrepancies including the adsorbing abilities and collecting power.
4 Conclusions
1) Micro-flotation tests showed that the flotation behaviors of DRN, DEN and DPN on kaolinite were different from each other. The collecting power of three collectors followed the order of DEN > DPN > DRN.
2) Based on the zeta potentials measurement, the surface potential of kaolinite was increased after reacting with DRN, DEN and DPN. The recruitments of zeta potentials aroused by three amines followed the order of DEN > DPN > DRN.
3) The results of dynamics simulation showed that the shapes of substituent groups bonding to N are deflected and twisted, and some bond angles are changed when the interactions of tertiary amines cations on kaolinite (001) surface take place.
4) After DRN12H+, DEN12H+ and DPN12H+ adsorb stably on kaolinite (001) surface, the distances of N atom away from (001) surface are different from each other. On the basis of Coulomb’s formula, the electrostatic forces between three tertiary amines cations and 4×4×3 kaolinite (001) surface are 1.38×10-7 N (DRN12H+), 1.44 ×10-6 N (DEN12H+) and 1.383 ×10-6 N (DPN12H+).
References
[1] LIU Xiao-wen, HU Yue-hua, JIANG Hao, CAO Xue-feng, QIU Guan-zhou. Crystal structure and surface property of kaolinite in hard kaolins and soft kaolins [J]. The Chinese Journal of Nonferrous Metals, 2001, 11(4): 684-687. (in Chinese)
[2] HU Yue-hua, LIU Xiao-wen, QIU Guan-zhou, HUANG Sheng-sheng. Solution chemistry of flotation separation of diaspore-type bauxite (I): Crystal structure and floatability [J]. Mining and Metallurgical Engineering, 2000, 20(2): 11-14. (in Chinese)
[3] LIU Xiao-wen, HU Yue-hua, XU Zheng-he. Effect of chemical composition on electrokinetics of diaspore [J]. Journal of Colloid and Interface Science, 2003, 11: 211-216.
[4] LIU Xiao-wen, HU Yue-hua, HUANG Sheng-sheng, QIU Guan-zhou. Chemical composition and surface property of kaolines [J]. Acta Mineralogica Sinica, 2001, 21(3): 443-447. (in Chinese)
[5] LIU Xiao-wen, HU Yue-hua, QIU Guan-zhou, HUANG Sheng-sheng. Study on wet-ability of the three dioctahedral phyllosilicate minerals [J]. Journal of Mineral Petrol, 2005, 25 (1): 10-13. (in Chinese)
[6] HU Yue-hua, WANG Yu-hua, WANG Dian-zuo. Flotation chemistry of aluminum and silicate and de-silication of bauxite [M]. Beijing: Science Press, 2004. (in Chinese)
[7] SUN Chuan-yao, YIN Wan-zhong. Flotation theory of silicate minerals [M]. Beijing: Science Press, 2001. (in Chinese)
[8] JIA Mu-xin, SUN Chuan-yao. Flotation and surface characteristics of some silicate minerals and its crystal chemistry analysis [J]. Conservation and Utilization of Mineral Resources, 2001, 10: 25-29. (in Chinese)
[9] ZHAO Shi-ming, WANG Dian-zuo, HU Yue-hua. The flotation behaviors of N-(3-aminopropyl)-dodecanamide on three aluminosilicates [J]. Minerals Engineering, 2003, 16: 1391-1395.
[10] ZHONG Hong, LIU Guang-yi, XIA Liu-yin. Flotation separation of diaspore from kaolinite, pyrophyllite and illite using three cationic collectors [J]. Minerals Engineering, 2008, 21: 1055-1061.
[11] WANG Yu-hua, HU Yue-hua, HE Ping-bo, GU Guo-hua. Reverse flotation for removal of silicates from diasporic-bauxite [J]. Minerals Engineering, 2004, 17: 63-68.
[12] XIA Liu-yin, ZHONG Hong, LIU Guang-yi, HUANG Zi-qing, CHANG Qin-wen. Flotation separation of the aluminosilicates from diaspore by a Gemini cationic collector [J]. Int J Miner Process, 2009, 92: 74-83.
[13] MASSOLA C P, CHAVES A P, LIMA J R B, ANDRADE C F. Separation of silica from bauxite via froth flotation[J]. Minerals Engineering, 2009, 22: 315-318.
[14] LI Hai-pu, HU Yue-hua, WANG Dian-zuo, XU Jing. Mechanism of interaction between cationic surfactant and kaolinite [J]. Journal of Central South University: Natural Science, 2004, 35(2): 228-233. (in Chinese)
[15] HU Yue-hua, SUN Wei, JIANG Hao, MILLER J D, FA Ke-qing. The anomalous behaviors of kaolinite flotation with dodecyl amine collector as explained from crystal structure considerations [J]. Int J Miner Process, 2005, 76: 163-172.
[16] LIU Guang-yi, ZHONG Hong, HU Yue-hua, ZHAO Sheng-gui, XIA Liu-yin. The role of cationic polyacrylamide in the reverse flotation of diasporic bauxite [J]. Minerals Engineering, 2007, 20: 1191-1199.
[17] LIU Chang-miao, HU Yue-hua, CAO Xue-feng. Substituent effects in kaolinite flotation using dodecyl tertiary amines [J]. Minerals Engineering, 2009, 22: 849-852.
[18] LI Shu-wen, FAN Ru-lin. Practical handbook of organic chemistry [M]. Shanghai: Shanghai Technique Press, 1981. (in Chinese)
[19] JIANG Hao, HU Yue-hua, QIN Wen-qing, QIU Guan-zhou. Interaction and flotation of diaspore with alkylamine hydrochlorides [J]. Transactions of Nonferrous Metals Society of China, 2001, 11: 430-433.
[20] CAO Xue-feng, HU Yue-hua. Synthesis of γ-alkoxy-propylamines and their collecting properties on aluminosilicate minerals[J]. Journal of Central South University of Technology, 2004, 11(3): 280-285.
[21] SONG Ming-yu. College physics [M]. Beijing: Tsinghua University Press, 2009: 10. (in Chinese)
叔胺在高岭石(001)面吸附的分子动力学模拟
刘长淼1, 2,冯安生1, 2,郭珍旭1, 2,曹学锋3,胡岳华3
1. 中国地质科学院郑州矿产综合利用研究所,郑州 450006;
2. 国家非金属矿资源综合利用工程技术研究中心,郑州 450006;
3. 中南大学 资源加工与生物工程学院,长沙 410083
摘 要:
三种叔胺(DRN,DEN,DPN)对高岭石的捕收能力顺序为:DEN>DPN>DRN。Zeta电位测试表明,这三种叔胺能显著增大高岭石的表面电位,对表面电位的增大同样遵循顺序:DEN>DPN>DRN。分子动力学模拟研究表明,叔胺阳离子在吸附到高岭石(001)面的过程中,N原子上所连接的取代基的空间形状发生了偏转或扭曲,相关键角发生了变化。结合量子化学计算结果进行综合计算可知,这三种叔胺阳离子与高岭石4×4×3的(001)面的库仑静电力分别为:1.38×10-7 N (DRN12H+),1.44×10-6 N (DEN12H+),1.383×10-6 N (DPN12H+)。
关键词:
(Edited by YUAN Sai-qian)
Foundation item: Project (2005CB623701) supported by the National Basic Research Program of China; Project (201011031) supported by National Department Public Benefit Research Foundation from Ministry of Land and Resources of China; Project (2935) supported by the Foundation for the Author of Zhengzhou Institute of Multipurpose Utilization of Mineral Resources CAGS, China; Project (1212011120304) supported by the Geological Surrey Program
Corresponding author: LIU Chang-miao; Tel: +86-13674908902; E-mail: changmiaoliu@163.com
DOI: 10.1016/S1003-6326(11)60944-8


