LiNi0.2Li0.2Mn0.6O2正极材料的合成与碳包覆
刘云建1, 2, 3,王亚平1,刘三兵2,吕军1,陈龙3,陈效华2
(1. 江苏大学 材料科学与工程学院,江苏 镇江,212013;
2. 奇瑞汽车博士后科研工作站,安徽 芜湖,241006;
3. 江苏大学 汽车与交通学院,江苏 镇江,212013)
摘 要:
温固相法合成LiNi0.2Li0.2Mn0.6O2固溶体正极材料,并通过球磨-低温热解对LiNi0.2Li0.2Mn0.6O2进行碳包覆;通过XRD,SEM和TEM对包覆前后的样品进行分析和表征。结果表明:球磨包覆前后样品具有层状固溶体结构,但包覆后颗粒粒径有所减小;包覆后LiNi0.2Li0.2Mn0.6O2 0.1C的放电比容量由包覆前的219 mA·h/g增加到246 mA·h/g,5C的放电比容量由包覆前的60 mA·h/g增加到包覆后的125 mA·h/g。50次循环后容量保持率由94.7%提高至97.8%。包覆后正极材料电荷转移阻抗从原来的62 Ω减小至37 Ω。
关键词:
锂离子电池;LiNi0.2Li0.2Mn0.6O2;碳包覆;电化学性能;倍率性能;
中图分类号:TM912 文献标志码:A 文章编号:1672-7207(2013)02-0482-05
Synthesized and carbon-coating of LiNi0.2Li0.2Mn0.6O2 cathodes
LIUYunjian1, 2, 3, WANG Yaping1, LIU Sanbing2, L Jun1, CHEN Long3, CHEN Xiaohua2
Jun1, CHEN Long3, CHEN Xiaohua2
(1. College of Material Science and Technology Jiangsu University, Zhenjiang 212013, China;
2. Postdoctoral Workstation of Chery Automobile Co., Ltd, Wuhu 241006, China;
3. School of Automobile and Traffic Engineering, Jiangsu University, Zhenjiang 212013, China)
Abstract: Li1.2Ni0.2Mn0.6O2 was synthesized by co-precipitation and high-temperature solid-state reaction. The carbon-coated Li1.2Ni0.2Mn0.6O2 was synthesized by ball-milling and low-temperature pyrogenation. The samples were characterized by XRD, SEM and TEM, respectively. The results show that the samples have layered structure, and the particle size diminishes after coating. The first discharge capacity at 0.1C is increased from 219 mA·h/g to 246 mA·h/g after coating. The discharge capacity at 5.0C is still 125 mA·h/g, while that of pristine is only about 60 mA·h/g. The capacity retaining ratio is increased from 94.7% to 97.8%. The charge transfer resistance is greatly reduced from 62 Ω to 37 Ω after carbon coatimg.
Key words: Li-ion battery; LiNi0.2Li0.2Mn0.6O2; carbon coated; electrochemical performance; rate performance
锂离子电池自从20世纪90年代问世以来,以其能量密度高,循环性能好、对环境有好和使用寿命长等优点,迅速被人们所接受,并得到了飞速的发展。随着社会的进步,人们对锂离子电池提出了更高的要求。目前常用的几种正极材料,如LiMn2O4,LiFePO4和LiNi1/3Mn1/3Co1/3O2已经不能满足锂离子动力电池特别是电动汽车用锂离子电池的要求[1-3]。近年来,有研究者发现Li2MnO3·LiMO2(M=Ni, Co, Mn)组成的固溶体材料具有比容量高、能量密度高、循环性能好和安全性好等优点,并很有希望成为下一代的锂离子动力电池正极材料[4-8]。目前研究证实,当充电电压为4.5 V时,Li2O从Li2MnO3中脱出,从而使材料表现出较高的放电比容量。并且在充放电过程中,MnO2保持骨架不变,保证了材料结构的稳定性[9-10]。但是,该材料也存在首次库仑效率较低,倍率性能不佳等缺点[11, 8]。Kang等[8]报道了利用Li-Ni-PO4包覆方法,有效地提高了0.5Li2MnO3·0.5LiNi0.44Co0.25Mn0.31O2正极材料的倍率性能。碳包覆是提高正极材料倍率性能的有效方法,目前已经广泛应用于锂离子电池正极材料的制备[12-14]。Liu等[15]采用真空热解法对Li[Li0.2Mn0.54Ni0.13Co0.13]O2正极材料进行碳包覆,取得了显著的效果。但是,该方法过程复杂,对设备要求高,不适合大规模工业生产。本研究采用通过简单的适合工业生产的机械球磨-低温裂解方法对LiNi0.2Li0.2Mn0.6O2进行碳包覆,研究碳包覆对正极材料电化学性能特别是倍率性能的影响,并对相关机理进行分析。
1 实验
1.1 材料制备与表征
按照一定物质的量比称取 NiSO4·6H2O (分析纯)、MnSO4·H2O(分析纯)和LiOH·H2O(分析纯) 3种物质。其中金属元素Ni和Mn的物质的量比n(Ni):n(Mn)=1:3,氢氧化锂与总的金属元素的物质的量比为1:2。氢氧化锂适当过量可以确保金属离子沉淀完全。将2种金属硫酸盐溶于去离子水,所得溶液记为A溶液,浓度为0.1 mol/L;氢氧化锂溶于去离子水得B溶液,浓度为0.1 mol/L。将B溶液缓慢滴加到A溶液中,同时剧烈搅拌,并控制溶液温度为70 ℃,pH为11.0~12.0。滴加完B溶液后再继续搅拌2 h,静置5 h后抽滤,并以去离子水洗涤3次获得镍锰复合氢氧化物。经120 ℃ 鼓风干燥箱中干燥2 h得到Ni0.25Mn0.75(OH)2粉末。
将Li2CO3和Ni0.25Mn0.75(OH)2按LiNi0.2Li0.2Mn0.6O2的化学计量比混合球磨,然后,在空气中于500 ℃预烧5 h,于900 ℃煅烧12 h,最后随炉冷却至室温,得到固溶体正极材料。
称取一定质量的酚醛树脂溶于酒精中,然后将制备的LiNi0.2Li0.2Mn0.6O2粉末加入其中并在球磨机中湿磨6 h后干燥。最后将混合粉末在空气气氛下于400 ℃加热1.5 h,得到最终的碳包覆LiNi0.2Li0.2Mn0.6O2正极材料。正极材料中碳的含量用碳硫分析仪测定。
对所得粉体材料进行XRD结构表征,仪器为D/max2A型转靶X线衍射仪(日本理学)。以Cu Kα为辐射源,电压为40 kV,电流为300 mA,步幅宽度为0.02°,扫描速度为2(°)/min,扫描范围2θ为10°~90°。
采用JEOL公司的JSM5600LV扫描电镜观察样品的表面形貌,操作电压为15 kV。用透射电镜(TEM,Tecnai G220 ST型透射电子显微镜,荷兰)观测颗粒微观形貌。
1.2 电池制作与测试
按照质量比为 8:1:1称量正极材料,导电碳黑与PVDF,在溶剂 NMP中混合成均匀浆料,均匀涂布于铝箔上。在80℃的真空箱中干燥制成正极片。在充满氩气的手套箱中装配成 CR2025 型扣式电池:以锂片为负极,Cellgard2400 聚丙烯多孔膜为隔膜,以溶解于EC/EMC/DMC(体积比为1:1:1)的1 mol/L LiPF6为电解液。在常温下,采用电池自动充放电仪(武汉蓝电)进行充放电及循环性能测试。充放电采用恒流/恒压方法,电池的测试电压范围为2.0~4.8 V。首次充放电电流为0.1C(其中,C为放电比容量),循环性能测试电流采用0.2C。
交流阻抗测试采用三电极体系进行,辅助电极和参比电极均采用金属锂片。测试仪器为上海辰华的CHI660D电化学工作站,交流阻抗的测试频率为0.01~100 000 Hz。
2 结果与讨论
图1所示为包覆前后LiNi0.2Li0.2Mn0.6O2的X线衍射图。从图1可以看出:材料属于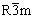 空间群,具有α-NaFeO2构型,峰(003)与(004)的强度比I003/I104大于1.2,且(018)和(110)峰分裂明显,说明富锂正极材料层状结构良好;在20°~25°之间出现一组较小的衍射峰,这是由于Li2MnO3的过渡金属层由n(Li)/n(Mn)= 1/2排列,具有超晶格有序性[16]。而包覆后Li2MnO3的特征峰强度有所减弱,表明超晶格有序程度减弱。
空间群,具有α-NaFeO2构型,峰(003)与(004)的强度比I003/I104大于1.2,且(018)和(110)峰分裂明显,说明富锂正极材料层状结构良好;在20°~25°之间出现一组较小的衍射峰,这是由于Li2MnO3的过渡金属层由n(Li)/n(Mn)= 1/2排列,具有超晶格有序性[16]。而包覆后Li2MnO3的特征峰强度有所减弱,表明超晶格有序程度减弱。
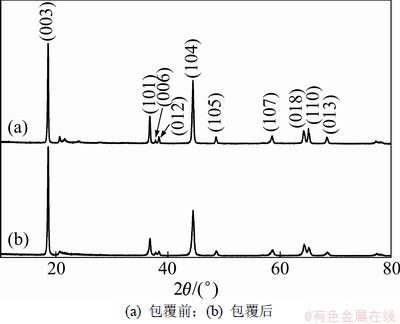
图1 包覆前后LiNi0.2Li0.2Mn0.6O2样品的XRD图谱
Fig.1 XRD pattern of LiNi0.2Li0.2Mn0.6O2 and carbon coated LiNi0.2Li0.2Mn0.6O2
图2所示为包覆前后样品的扫描电镜图,从图2可以看出:包覆前样品表面光滑,粒径为8~10 μm。而经过球磨包覆后,表面出现一层絮状包覆物,并且粒径也变成3~5 μm。较小的颗粒粒径缩短了Li+在正极材料颗粒内部的扩散路径,有利于提高正极材料的电化学性能,特别是倍率性能[17]。经过碳硫分析仪测定,包覆后的样品碳的质量分数为3.2%。
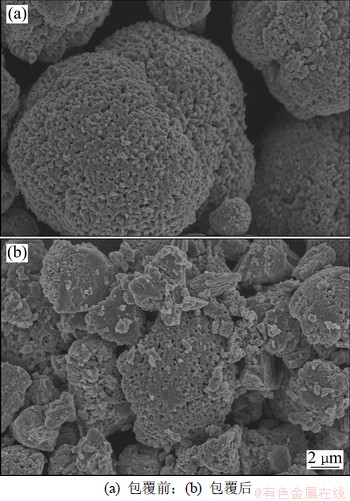
图2 包覆前后LiNi0.2Li0.2Mn0.6O2样品的SEM图
Fig.2 Scanning electron microscopy (SEM) images of LiNi0.2Li0.2Mn0.6O2
图3所示为包覆前后LiNi0.2Li0.2Mn0.6O2样品的透射电镜图。从图3可以看出:包覆前样品表面光滑;经过球磨-碳裂解后,碳均匀地包覆在LiNi0.2Li0.2Mn0.6O2颗粒表面。碳包覆后对正极材料可以起到以下作用:(1) 碳能够有效地阻隔电解液和正极材料的直接接触,减少电解液对正极材料的侵蚀;(2) 碳具有良好的导电性,能够有效地提升正极材料的倍率性能[12]。
图4所示为包覆前后LiNi0.2Li0.2Mn0.6O2样品的首次充放电曲线,充放电区间为2.0~4.8 V,充放电电流为0.1C。从图4可以看出:包覆前后样品的充电曲线中在4.5 V处都出现了1个平台,该平台代表Li2O从Li2MnO3脱出反应[8]。在放电曲线中,低于3.5 V的平台则代表Mn4+被还原为Mn3+反应[18]。包覆前后的LiNi0.2Li0.2Mn0.6O2样品首次放电容量分别为219和246 mA·h/g。该结果表明:经过碳包覆后,LiNi0.2Li0.2Mn0.6O2的首次放电容量得到了明显提升。这可能是因为:(1) 表面包覆的碳层,有效地提高了电极材料的导电性,有利于锂离子的迁移;(2) 碳包覆有效地抑制了电解液和正极材料之间的反应,减少正极表面SEI膜生成时所消耗的活性锂,进而使包覆后的样品表现出较高的放电比容量。
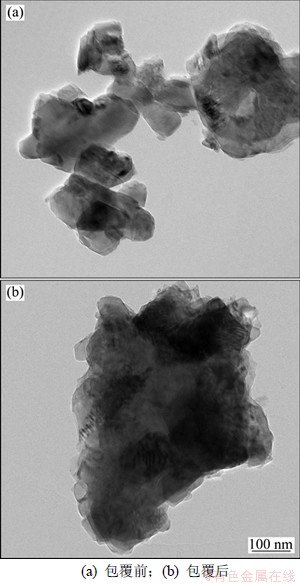
图3 包覆前后LiNi0.2Li0.2Mn0.6O2样品的TEM图
Fig.3 Transmission electron microscopy (TEM) images of LiNi0.2Li0.2Mn0.6O2 and carbon coated LiNi0.2Li0.2Mn0.6O2
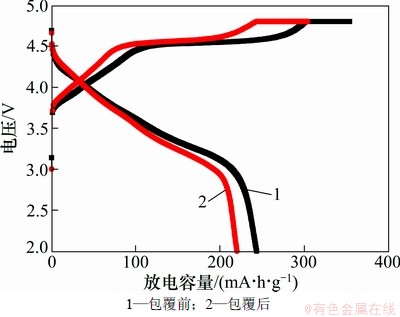
图4 LiNi0.2Li0.2Mn0.6O2样品的首次放电曲线
Fig.4 First discharge curves of Li1Ni0.2Li0.2Mn0.6O2
图5所示为包覆前后LiNi0.2Li0.2Mn0.6O2样品的不同倍率循环性能图。样品的充电电流均为0.1 C,而放电电流分别为0.1C,0.2C,0.5C,1C,2C和5C。从图5可以看出:随着放电倍率的增大,样品的放电比容量均有所降低,但是,包覆前后样品的放电容量差逐渐增大。例如,0.1C放电时,包覆前后的放电比容量分别为219和246 mA·h/g;1C放电时包覆前后的放电比容量分别为100和160 mA·h/g;当放电比容量增大至5C时,包覆前后的放电比容量分别为60和125 mA·h/g。该结果表明:通过机械球磨-碳裂解的包覆方式有效地提高了LiNi0.2Li0.2Mn0.6O2的倍率放电性能,特别是大倍率下的放电比容量。造成该现象的原因可能为:(1) 碳包覆提高了LiNi0.2Li0.2Mn0.6O2正极材料的导电性;(2) 经过球磨包覆后,形成了较小的颗粒,缩短了锂离子的扩散路径;(3) 碳包覆能够有效地抑制正极材料表面SEI膜生成和并减小其厚度,进而有利于锂离子的扩散[15]。
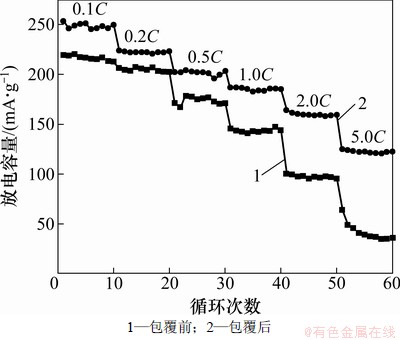
图5 LiNi0.2Li0.2Mn0.6O2样品的不同倍率循环性能
Fig.5 Cycling performances of LiNi0.2Li0.2Mn0.6O2 with different rates
图6所示为包覆前后LiNi0.2Li0.2Mn0.6O2样品的循环性能图,充放电电流为0.2C。包覆前后LiNi0.2Li0.2Mn0.6O2样品0.2C的放电容量分别为206和223 mA·h/g。50次循环以后放电容量为195和218 mA·h/g,容量保持率分别为94.7%和97.8%。该结果表明:碳包覆有效地改善了LiNi0.2Li0.2Mn0.6O2正极材料的循环性能。这可能是因为LiNi0.2Li0.2Mn0.6O2正极材料表面包覆的碳层,有效地减少了电解液和LiNi0.2Li0.2Mn0.6O2的接触面积,抑制了LiNi0.2Li0.2Mn0.6O2电解液的反应,进而改善了LiNi0.2Li0.2Mn0.6O2的循环性能。
图7所示为包覆前后LiNi0.2Li0.2Mn0.6O2的交流阻抗图,测试前LiNi0.2Li0.2Mn0.6O2电极的电压稳定在4.3 V左右。包覆前后LiNi0.2Li0.2Mn0.6O2阻抗图谱均由高频区的半圆和低频区的直线组成。高频区的半圆弧代表正极材料表面电荷转移阻抗Rct;低频区的直线代表Li+在固相活性物质中的扩散Warburg阻抗Zw[19]。经过Zview 软件模拟得知:包覆前后的LiNi0.2Li0.2Mn0.6O2电极的电荷转移阻抗分别为62 Ω和37 Ω。该结果表明:包覆后LiNi0.2Li0.2Mn0.6O2电极表面的电荷转移阻抗明显减小。这是因为碳包覆有效地抑制了正极材料和电解液的反应,减少了SEI膜的生成;其次,碳包覆提高了电极的导电率,进而降低了正极材料的电荷转移阻抗,因此表现出良好的倍率性能。通过简单的机械球磨-碳裂解方法,有效地提高了LiNi0.2Li0.2Mn0.6O2正极材料的首次放电比容量、循环性能和倍率性能。
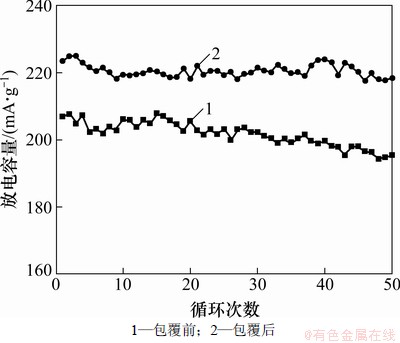
图6 LiNi0.2Li0.2Mn0.6O2样品的循环性能图
Fig.6 Cycling performance of LiNi0.2Li0.2Mn0.6O2
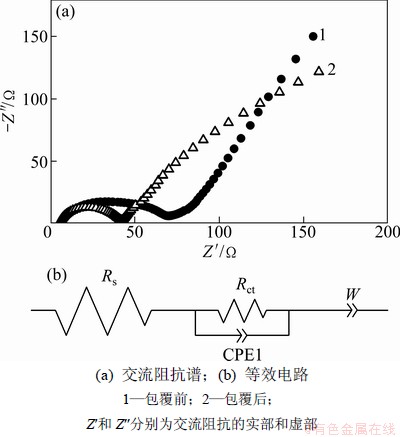
图7 LiNi0.2Li0.2Mn0.6O2样品的交流阻抗图谱和模拟等效电路图
Fig.7 AC impedence of LiNi0.2Li0.2Mn0.6O2 and equivalent circuit model
3 结论
(1) 通过共沉淀-高温固相法和球磨-低温热解法合成了LiNi0.2Li0.2Mn0.6O2和碳包覆的LiNi0.2Li0.2Mn0.6O2固溶体正极材料。包覆前后,Li1.2Ni0.2Mn0.6O2均保持良好的层状结构;经过球磨包覆后,碳均匀的包覆在正极材料表面,正极材料的颗粒粒径有所减小。
(2) LiNi0.2Li0.2Mn0.6O2的首次放电容量由包覆前的219 mA·h/g增加到包覆后的246 mA·h/g。倍率性能得到明显提升,5C的放电比容量由包覆前的60 mA·h/g增加到包覆后的125 mA·h/g。50次循容量保持率由94.7%提高至97.8 %。
(3) 包覆后正极材料的电荷转移阻抗由原先的62 Ω减小至37 Ω。较高的导电率、较小的粒径以及较小的电荷转移阻抗是碳球磨-碳包覆后电化学性能得到改善的原因。
参考文献:
[1] LIU Bing, ZHANG Qian, HE Shici, et al. Improved electrochemical properties of Li1.2Ni0.18Mn0.59Co0.03O2 by surface modification with LiCoPO4[J]. Electrochimica Acta, 2011, 56(19): 6748-6751.
[2] Taniguchi L. Powder properties of partially substituted LiMxMn2-xO4(M=AI, Cr, Fe and Co) synthesized by ultrasonies pray pyrolysis[J]. Materials Chemistry and Physies, 2005, 92(1): 172-175.
[3] Mohamed A, Jose M A, Rosa Rojas M, et al. Sub-micrometric LiCr0.2Ni0.4Mn1.4O4 spinel as 5 V-cathode material exhibiting huge rate capability at 25 and 55 ℃[J]. Electrochemistry Communications, 2010, 12(4): 548-552.
[4] Kim D H, Kang S H, Mahalingam B, et al. High-energy and high-power Li-rich nickel manganese oxide electrode materials[J]. Electrochemistry Communications, 2010, 12(10): 1618-1621.
[5] Jeong J H, Jin B S, Kim W S, et al. The influence of compositional change of 0.3Li2MnO3·0.7LiMn1-xNiyCo0.1O2 (0.2≤x≤0.5, y=x-0.1) cathode materials prepared by co-precipitation[J]. Journal of Power Sources, 2011, 196(11): 3439-3442.
[6] Sivaprakash S, Majumder S B. Spectroscopic analyses of 0.5Li[Ni0.8Co0.15Zr0.05]O2-0.5Li[Li1/3Mn2/3]O2 composite cathodes for lithium rechargeable batteries[J]. Solid State Ionics, 2010, 181(5): 730-739.
[7] Koening G M, Belharouak J I, Wu H M, et al. Hollow lithiated metal oxide particles as lithium-ion battery cathode materials[J]. Electrochimica Acta, 2011, 56(11): 1426-1431.
[8] Kang S H, Thackeray M M. Enhancing the rate capability of high capacity xLi2MnO3·(1-x)LiMO2(M=Mn, Ni, Co) electrodes by Li-Ni-PO4 treatment[J]. Electrochemistry Communications, 2009, 11(3): 748-751.
[9] Park S H, Kang S H, Johnson C S, et al. Lithium-manganese-nickel-oxide electrodes with integ rated layered-spinel structures for lithium batteries[J]. Electrochemistry Communications, 2007, 9(1): 262-268.
[10] Johnson C S, Li N, Vaughey J T, et al. Lithium-manganese oxide electrodes with layered-spinel composite structures xLi2MnO3·(1-x)Li1+yMn2-yO4 (0<x<1, 0≤y≤0.33) for lithium batteries[J]. Electrochemistry Communications, 2005, 7(1): 528-536.
[11] Zheng J M, Zhang Z R, Wu X B, et al. The effects of AlF3 coating on the performance of Li[Li0.2Mn0.54Ni0.13Co0.13]O2 positive electrode material for lithium-ion battery[J]. Journal of Electrochemical Society A, 2008, 155(10): 775-782.
[12] CHENG Fuquan, WANG Wan, ZHOU Tan, et al. High power performance of nano-LiFePO4/C cathode material synthesized via lauric acid-assisted solid-state reaction[J]. Electrochimica Acta, 2011, 56(8): 2999-3005.
[13] Wang L, Liang G C, Qu X Q, et al. Effect of synthesis temperature on the properties of LiFePO4/C composites prepared by carbothermal reduction[J].Journal of Power Sources, 2009, 189(1): 423-428.
[14] Yang T Y, Zhang N Q, Lang Y, et al. Enhanced rate performance of carbon-coated LiNi0.5Mn1.5O4 cathode material for lithium ion batteries[J]. Electrochimica Acta, 2011, 56(11): 4058-4064.
[15] Liu J, Wang Q Y, Jayan B R, et al. Carbon-coated high capacity layered Li[Li0.2Mn0.54Ni0.13Co0.13]O2 cathodes[J]. Electro- chemistry Communications, 2010, 12(2): 750-753.
[16] Lu Z H, Chen Z H, Dahn J R. Lack of cation clustering in Li[NixLi1/3-2x/3Mn2/3-x/3]O2 (0≤x≤1/2) and Li[CrxLi(1-x)/3Mn(2-2x)/3]O2 (0<x<1)[J]. Chemistry of Materials, 2003, 15(16): 3214-3220.
[17] ZHENG Jianming, WU Xuebin, YANG Yong. A comparison of preparation method on the electrochemical performance of cathode material Li[Li0.2Mn0.54Ni0.13Co0.13]O2 for lithium ion battery[J]. Electrochimica Acta, 2011, 56 (8): 3071-3078.
[18] YU Lingyan, QIU Weihua, LIAN Fang. Comparative study of layered 0.65Li[Li1/3Mn2/3]O2·0.35LiMO2 (M=Co, Ni1/2Mn1/2 and Ni1/3Co1/3Mn1/3) cathode materials[J]. Materials Letters, 2008, 62(17/18): 3010-3013.
[19] Arira P, Popov B N, White R E. Electrochemical investigations of cobalt doped LiMn2O4 as cathode material for lithium ion batteries[J]. Journal of Electrochemical Society, 1998, 145(3): 807-8151.
(编辑 何运斌)
收稿日期:2012-04-08;修回日期:2012-06-02
基金项目:江苏省自然科学基金资助项目(BK2011530);江苏大学高级人才启动基金资助项目(10JDG041);江苏省博士后基金资助项目(1102121C)
通信作者:刘云建(1981-),男,江苏溧阳人,博士后,副教授,从事新能源材料及电化学研究;电话:13505282025;E-mail:lyjian122331@yahoo.com.cn
摘要:通过共沉淀-高温固相法合成LiNi0.2Li0.2Mn0.6O2固溶体正极材料,并通过球磨-低温热解对LiNi0.2Li0.2Mn0.6O2进行碳包覆;通过XRD,SEM和TEM对包覆前后的样品进行分析和表征。结果表明:球磨包覆前后样品具有层状固溶体结构,但包覆后颗粒粒径有所减小;包覆后LiNi0.2Li0.2Mn0.6O2 0.1C的放电比容量由包覆前的219 mA·h/g增加到246 mA·h/g,5C的放电比容量由包覆前的60 mA·h/g增加到包覆后的125 mA·h/g。50次循环后容量保持率由94.7%提高至97.8%。包覆后正极材料电荷转移阻抗从原来的62 Ω减小至37 Ω。


