
Improved hydrogenation properties of Mg-x(Ti0.9 Zr0.2 Mn1.5 Cr0.3) composites
FAN Mei-qiang(范美强)1, 2, SUN Li-xian(孙立贤)1, XU Fen(徐 芬)3
1. Materials and Thermochemistry Laboratory, Dalian Institute of Chemical Physics,
Chinese Academy of Sciences, Dalian 116023, China;
2. Department of Materials Science and Engineering, China Jiliang University, Hangzhou 310018, China;
3. College of Chemistry and Chemical Engineering, Liaoning Normal University, Dalian 116029, China
Received 20 July 2009; accepted 20 April 2010
Abstract:
Mg-x(Ti0.9 Zr0.2 Mn1.5 Cr0.3)(x=20%, 30%, 40%) (mass fraction) composite powders were prepared by reactive ball milling with hydrogen and their hydrogen storage properties and microstructure were investigated by XRD, SEM and pressure- composition-temperature measurement. The results show that the composites have 3.83%-5.07% hydrogen capacity at 553 K and good hydrogenation kinetics, even at room temperature. Among them, the milled Mg-30%(Ti0.9Zr0.2Mn1.5Cr0.3) composite has the highest hydrogenation kinetics as it can quickly absorb 2.1% hydrogen at 373 K, 3.5% in 2 000 s at 473 K, even 3.26% in 60 s at 553 K under 3 MPa hydrogen pressure. The improved hydrogenation properties come from the catalytic effect of Ti0.9 Zr0.2 Mn1.5 Cr0.3 particles dispersed uniformly on the surface of Mg particles.
Key words:
hydrogen storage; hydrogenation kinetic; Ti0.9 Zr0.2 Mn1.5 Cr0.3 alloy; catalytic effect;
1 Introduction
Magnesium and Mg-based hydrogen storage alloys have some advantages of high hydrogen storage capacity (7.6% for pure magnesium, mass fraction), light weight and low cost, so they are considered to be potential materials for hydrogen storage[1-2]. However, there are still some problems such as the high decomposition temperature[3] and sluggish hydrogenation/ dehydrogenation kinetics which limit their practical application for hydrogen storage materials. Many effective methods have been explored to improve the dehydrogenation-hydrogenation kinetics of Mg-based hydrogen storage materials via preparing milled Mg composites with transition mental[4-5], transition mental oxide[6-7] or a typical hydrogen storage alloy[8-9]. Among these methods, hydrogen storage alloys were regarded as the most potential additives, which not only accelerated the hydrogenation kinetic, but also increased the hydrogen capacity of Mg composites. Compared with the milled Mg metal, the milled Mg-30%(Ti1V1.1Mn0.9) composite[9] had better hydrogen properties of 4.46% hydrogen at 606 K under the atmosphere of 3 MPa hydrogen in 1 h and 1.77% hydrogen within 1 h even at 377 K. The improved hydrogen properties were related to the catalytic effect of Ti1V1.1Mn0.9 alloy which was dispersed uniformly on the surface of Mg particles and provided the pathways for the hydrogen diffusion into Mg composites. However, there is still no consideration of the additive content on the activation energy of Mg composites.
In this work, the good hydrogen storage properties of Ti0.9Zr0.2Mn1.5Cr0.3 alloy were summarized in Ref.[10]. From the view point of improving the hydrogen storage properties, we prepared the milled Mg-x(Ti0.9Zr0.2- Mn1.5Cr0.3)(x=20%, 30%, 40%) composites and examined their hydrogenation kinetics. The relationships between the additive content and hydrogen storage properties were also investigated from hydrogenation kinetics at different temperatures and the activation energy. The aim of this work is to find an excellent hydrogen storage material and explore the role of the Ti0.9Zr0.2Mn1.5Cr0.3 in Mg-based composite.
2 Experimental
The Ti0.9Zr0.2Mn1.5Cr0.3 alloy was prepared by vacuum magnetic levitation melting under argon atmosphere, according to Ref.[10]. The ingots were turned over and remelted for three times to insure the homogeneity. The ingots were mechanically crushed and ground into the powder of 74 μm in size through ball milling treatment.
The Mg powder (99.5%) and the Ti0.9Zr0.2Mn1.5Cr0.3 alloy powder were mixed homogenously with a composition of Mg-x(Ti0.9Zr0.2Mn1.5Cr0.3) (x=20%, 30%, 40%). The mixture was milled under 0.3 MPa H2 atmosphere with QM-ISP planetary ball miller at a rotation speed of 450 r/min for 20 h. Ball-to-powder mass ratio was 30?1.
Powder X-ray diffraction (XRD) studies were carried out on a DRON-2 diffractometer (Crystalline silicon is the internal standard). Microstructure studies (EDX analysis) were performed on a CAMEBAX- microbeam electron microprobe equipped with a KEVEX energy-dispersive analyzer. The hydrogenation kinetics of the composite were determined with the conventional Sievert-type P-C-T apparatus as described in our previous study[11]. 1.2 g alloy was measured and put in steel circular tube and the tube was loaded into a stainless steel reactor in argon box. The hydrogenation experiments were carried out under 3 MPa H2 with the purity of 99.999 9%. The experimental temperature was 298, 373, 473 and 553 K, respectively. The pressure- composition-temperature P-C-T measurements were carried out at 553 K and within the hydrogen pressure range of 0-3.5 MPa.
3 Results and discussion
3.1 Sample characterization
The XRD patterns of the milled Mg-30% (Ti0.9 Zr0.2- Mn1.5 Cr0.3) alloy are shown in Fig.1. The peaks of the hexagonal C14 Lares phase in Ti0.9Zr0.2Mn1.5Cr0.3 alloy still occurred in the XRD patterns of Mg-30% (Ti0.9Zr0.2Mn1.5Cr0.3) alloy. Meanwhile, peaks of MgH2 are observed except those of Mg. The results confirm that MgH2 is formed in the milling process under H2 atmosphere. Choosing the MgH2 (110) diffraction peak and Ti0.9 Zr0.2 Mn1.5 Cr0.3 alloy (201) diffraction peak, the evaluated values of the average particle size are calculated: MgH2, 15 nm; Ti0.9Zr0.2Mn1.5Cr0.3 alloy, 33 nm. The nano-structure of the composite is resulted from the accumulated defects and disorder in the milling process, reflecting the broadened peaks in the XRD pattern. After dehydrogenation-hydrogenation cycles, the evaluated value of the MgH2 average particle size
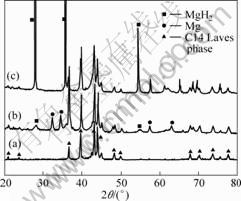
Fig.1 XRD patterns of alloys: (a) Ti0.9Zr0.2Mn1.5Cr0.3 alloy; (b) Mg-30%(Ti0.9Zr0.2Mn1.5Cr0.3) alloy before dehydrogenation- hydrogenation cycling; (c) Mg-30%(Ti0.9Zr0.2Mn1.5Cr0.3) alloy after dehydrogenation-hydrogenation cycling
increases quickly from 15 nm to 51 nm. But the evaluated average particle size of the Ti0.9Zr0.2Mn1.5Cr0.3 alloy decreases slightly from 33 nm to 28 nm. Peaks of MgH2 and C14 Laves phase structure dominate in the XRD patterns while peaks of Mg disappear.
Fig.2 further shows the SEM micrographs of Mg-30%(Ti0.9 Zr0.2 Mn1.5 Cr0.3) composite before and after dehydrogenation/hydrogenation cycling. The particle size is less than 1 μm after being milled for 20 h in H2 atmosphere, compared with the Mg particle of 40 μm size in Fig.2(a). This change corresponds to cumulative defects and disorder of both MgH2 and Ti0.9Zr0.2Mn1.5-Cr0.3, resulting in the reduction of crystallite size to a nano-structure. It can be imagined that the Ti0.9Zr0.2-Mn1.5Cr0.3 is embedded on the surface of Mg uniformly in the milling process as the Ti0.9Zr0.2Mn1.5Cr0.3 alloy is brittle and easy to be ground to the very tiny particles. So, it is hard to identify Ti0.9Zr0.2Mn1.5Cr0.3 particles in the milled Mg-30% (Ti0.9Zr0.2Mn1.5Cr0.3) composite. After dehydrogenation- hydrogenation cycling, the particle size increases distinctly and the particles accumulate together, as seen from Fig.2(b).
3.2. Pressure-composition-temperature analysis
Fig.3 presents the hydrogenation isotherm of Mg- x(Ti0.9Zr0.2Mn1.5Cr0.3) (x=20%, 30%, 40%) composites at 553 K. It can be seen that the Mg-x(Ti0.9Zr0.2Mn1.5Cr0.3) (x=20%, 30%, 40%) composites can absorb 3.83%- 5.07% hydrogen at 553 K. The hydrogen adsorption of the composite is proportional to the hydrogen pressure. The hydrogen capacity comes from two parts: one from the absorption of Mg and the other from the absorption of Ti0.9Zr0.2Mn1.5Cr0.3 alloy. At the lower hydrogen pressure of 0.4 MPa, the Mg-x(Ti0.9Zr0.2Mn1.5Cr0.3) (x=20%, 30%, 40%) composites have almost 0.5%
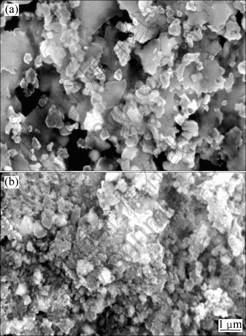
Fig.2 SEM micrographs of Mg-30%(Ti0.9Zr0.2Mn1.5Cr0.3) alloy before (a) and after (b) dehydrogenation-hydrogenation cycling
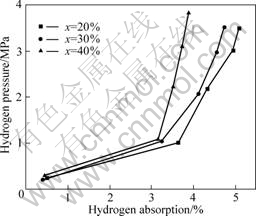
Fig.3 Hydrogenation isotherm of Mg-x(Ti0.9 Zr0.2 Mn1.5 Cr0.3) (x=20%, 30%, 40%) composites at 553K for one cycle (Each sample was dehydrogenated at 553 K for 10 h before collecting isotherm data)
hydrogen capacity which mainly comes from the absorption of the Ti0.9- Zr0.2Mn1.5Cr0.3 alloy. But at 1 MPa hydrogen pressure, the Mg-x(Ti0.9Zr0.2Mn1.5Cr0.3) (x=20%, 30%, 40%) composites can absorb 3.1%-3.6% hydrogen. We supposed Ti0.9Zr0.2-Mn1.5Cr0.3 were fully hydrogenated. Hence, the hydrogen absorption capacities contributed by Ti0.9Zr0.2Mn1.5Cr0.3 in the composites are 0.32%, 0.48% and 0.64%, respectively. Therefore, the residual 2.5%-3.3% hydrogen should be absorbed by Mg at 553 K. And with hydrogen pressure further increasing, Mg can absorb more hydrogen.
Fig.4 shows the hydrogenation kinetics of the milled Mg-x(Ti0.9Zr0.2Mn1.5Cr0.3) (x=20%, 30%, 40%) composites at 3 MPa hydrogen pressure and different temperatures. The composites have good hydrogenation kinetic and can absorb 0.37%-1.21% hydrogen in 2 000 s at 298 K. With temperature increasing, the hydrogenation kinetics become better and the composites absorb more hydrogen of 0.89%-2.02% at 373 K, 3.2%-3.52% at 473 K and 3.31%-3.71% at 553 K in 2 000 s, respectively. Among the composites, Mg-30(Ti0.9- Zr0.2 Mn1.5 Cr0.3) composite has the best hydrogen storage properties and absorbs 2.11% in 2 000 s at 373 K, 3.52% in 2 000 s at 473 K and 3.26% in 60 s at 553 K, as seen
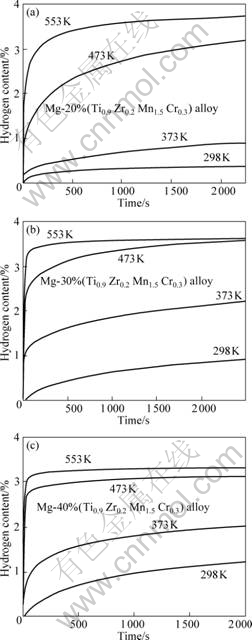
Fig.4 Hydrogenation kinetics of Mg-x(Ti0.9 Zr0.2 Mn1.5 Cr0.3) (x=20%, 30%, 40%) alloy at 3 MPa hydrogen and different temperatures
in Fig.4(b).
The Mg-x(Ti0.9Zr0.2Mn1.5Cr0.3) (x=20%, 30%, 40%) composites should have higher hydrogenation kinetics with the content increase of Ti0.9Zr0.2Mn1.5Cr0.3 alloy because of its catalytic effect and good hydrogenation kinetic. To confirm the above hydrothesis, we used the Arrhenius equation to calculate the activation energy of different composites:
k=Aexp[-Ea/(RT)] (1)
Eq.(1) gives the dependence of a rate constant (k) on the temperature (T) and the activation energy (Ea). By determining k from the hydrogenation rates obtained at different temperatures and using Eq.(1), the activation energies of the milled Mg-x(Ti0.9 Zr0.2 Mn1.5 Cr0.3) (x=20%, 30%, 40%) composites are calculated to be 18.012, 16.857 and 14.715 kJ/mol, respectively, from the Arrhenius plot of the reaction rate constant k against the reciprocal temperature in Fig.5. Obviously, content increase of Ti0.9Zr0.2Mn1.5Cr0.3 alloy favors to improve the hydrogenation kinetic of Mg-x(Ti0.9Zr0.2Mn1.5Cr0.3) (x=20%, 30%, 40%) composites, but reduce the hydrogen capacity of the Mg composites correspondingly. There is a tradeoff issue, so the rational content of Ti0.9Zr0.2Mn1.5Cr0.3 alloy should be pursued.
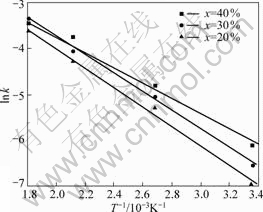
Fig.5 Arrhenius plots for hydrogenation rates of Mg- x(Ti0.9Zr0.2 Mn1.5 Cr0.3) (x=20%, 30%, 40%) composites
Generally, Mg could absorb hydrogen only at temperatures higher than 573 K with a slower hydrogenation rate. But in this work, the milled Mg- x(Ti0.9 Zr0.2 Mn1.5 Cr0.3) (x=20%, 30%, 40%) composites show the improved hydrogenation kinetics. They could absorb hydrogen rapidly at lower temperatures. The reasons are attributed to the following factors. Firstly, the Ti0.9Zr0.2Mn1.5Cr0..3 alloy powders are ground to very fine powders during high-energy ball milling. Mg is hydrogenated to produce MgH2 via the catalytic effect of the alloy in the milling process, and the same results could also be found in Ref.[12]. MgH2 is more brittle and easy to be broken to small particles, resulting in more fresh surface areas of Mg for hydrogenation. Furthermore, large amounts of defects could be generated in Mg in the milling process which act as active sites for hydrogenation[13]. The above reasons are favorable for improving the hydrogenation properties of Mg. Secondly, the additive phase Ti0.9Zr0.2Mn1.5Cr0.3 may catalyze the hydrogenation of Mg. Some studies[14-15] of MgH2-based composite found that the additive particles were dispersed on the surface of Mg matrix and acted as a hydrogen pump. In this study, the very fine Ti0.9 Zr0.2- Mn1.5 Cr0.3 particles were also embedded on the surface of Mg uniformly. During hydrogenation, hydrogen molecules were easily decomposed to hydrogen atoms on the surface of Ti0.9 Zr0.2 Mn1.5 Cr0.3 and finally absorbed in the alloy, leading to the formation of Ti0.9 Zr0.2 Mn1.5 Cr0.3 hydride:
2H2+Ti0.9Zr0.2Mn1.5Cr0.3→Ti0.9Zr0.2Mn1.5Cr0..3Hx (2)
When the hydrogen concentration was built up to a certain degree in the Ti0.9 Zr0.2 Mn1.5 Cr0.3 alloy, hydrogen would diffuse from Ti0.9 Zr0.2 Mn1.5 Cr0.3 into Mg through the Ti0.9 Zr0.2 Mn1.5 Cr0.3/Mg interfaces, resulting in the hydrogenation of Mg. Therefore, Ti0.9Zr0.2Mn1.5Cr0.3 alloy actually works as a hydrogen diffusion path and hydrogen pump in the hydrogenation of the composites.
4 Conclusions
1) The milled Mg-x(Ti0.9Zr0.2Mn1.5Cr0.3) (x=20%, 30%, 40%) composites have good hydrogenation properties, which have hydrogen capacity of 3.83%-5.07% at 553 K and good hydrogenation kinetic, even at room temperature.
2) Among the composites, the milled Mg-30%(Ti0.9Zr0.2Mn1.5Cr0.3) composites have high hydrogenation kinetics as it could quickly absorb 2.1% hydrogen at 373 K, 3.5% at 473 K, even 3.26% at 553 K under 3 MPa hydrogen pressure.
3) Via the XRD, SEM and P-C-I measurement, the improved hydrogen storage properties come from catalytic effect of the Ti0.9Zr0.2Mn1.5Cr0.3 alloy. It disperses uniformly in the composites, providing path for hydrogen diffusion through Mg matrix and decreasing the activation energy of the composite.
References
[1] IMAMURA H, MASANARI K, KUSUHARA M. High hydrogen storage capacity of nanosized magnesium synthesized by high energy ball-milling [J]. J Alloys Comp,2005, 386: 211-216.
[2] LI P. XU K D, CHOU K C. Kinetics of hydrogen absorption and desorption of a mechanically milled MgH2+5at% V nanocomposite [J]. J University of Science and Technology Beijing [J]. 2006, 13: 359-362.
[3] BOGDANOVIC B, HARTEIG T H, SPLIETHOFF B. The development, testing and optimization of energy storage materials based on the MgH2-Mg system [J]. Int of Hydrogen Energy, 1993, 18(7): 575-589.
[4] LI Song-lin, LIU Yil, VARIN R A, LIU Huai-fei, CUI Jian-min, CHEN Shi-qi. Effect of ball milling methods on synthesis and desorption properties of nanocrystalline mg2FeH6 hydrogen storage materials [J]. The Chinese Journal of Nonferrous Metals, 2008, 18(1): 42-47. (in Chinese)
[5] LI P, XU K D, CHOU K C. Kinetics of hydrogen absorption and desorption of a mechanically milled MgH2+5at% V nanocomposite [J]. J University of Science and Technology Beijing, 2006, 13: 359-362.
[6] WANG P, WANG A M, ZHANG H F. Hydrogenation characteristics of Mg–TiO2 (rutile) composite [J]. J Alloys Comp,2000, 313: 218-223.
[7] ARUEY-ZINSOU K F, FERNADEZ J R A, KLASSEN T. Effect of Nb2O5 on MgH2 properties during mechanical milling [J]. Int J Hydrogen Energy,2007, 32: 2400-2407.
[8] ZHAO Xian-jun, LI Qian, LIN Gen-wen, CHOU Kuo-chih, ZHANG Jie-yu, LU Xiong-gang. Hydriding reaction kinetics of Mg-Mg2Ni(1-x)Mex compositions [J]. The Chinese Journal of Nonferrous Metals, 2008, 18(5): 873-878. (in Chinese)
[9] GU H, ZHU Y F, LI L Q. Characterization of hydrogen storage properties of Mg-30% Ti1.0V1.1Mn0.9 composite [J]. J Alloys Compd, 2006, 424: 382-387.
[10] CHU H L, ZHANG Y, SUN L X. Structure, morphology and hydrogen storage properties of composites prepared by ball milling Ti0.9Zr0.2Mn1.5Cr0.3V0.3 with La–Mg-based alloy [J]. Int J Hydrogen Energy, 2007, 32: 3363-3369.
[11] ZHANG Y, ZHANG W S, WANG A Q. LiBH4 nanoparticles supported by disordered mesoporous carbon: Hydrogen storage performances and destabilization mechanisms [J]. Int J Hydrogen Energy, 2007, 32: 3976-3980.
[12] KANDAVEL M, RAMAPRABHU R. Correlation between hydrogen storage properties and amount of alloy particles in Mg-based composites [J]. J Alloys Comp, 2007, 438: 285-292.
[13] BOUOUDINA M, GRANT D, WALKER G. Review on hydrogen absorbing material-structure, microstructure, and thermodynamic properties [J]. Int J Hydrogen Energy, 2006, 31: 177-182.
[14] ORIMO S, FUJII H, HORIE S. Investigation of interfacial material design-the effect of interface microstructures in ZrCr1.8Cu0.3/Mg on MgH2 formation as a result of hydrogen interdiffusion [J]. J Alloys Compd, 1995, 231: 766-772.
[15] CASTRO J F R, SANTOS S F, COSTA A L M. Structural characterization and dehydrogenation behavior of Mg–5 at%Nb nano-composite processed by reactive milling [J]. J Alloys Comp, 2004,376: 251-256.
(Edited by LI Xiang-qun)
Foundation item:Projects(20833009, 20873148, U0734005) supported by the National Natural Science Foundation of China; Project(2010CB631303) supported by the National Basic Research Program of China; Project(2009A11GX052) supported by Dalian Science and Technology Foundation, China; Project(KFJJ10-1Z) supported by the State Key Laboratory of Explosion Science and Technology, Beijing Institute of Technology, China; Project(Y4090507) supported by the Zhejiang Basic Research Program of China
Corresponding author: SUN Li-xian; E-mail: lxsun@dicp.ac.cn
DOI: 10.1016/S1003-6326(09)60319-8


