J. Cent. South Univ. Technol. (2007)02-0236-07
DOI: 10.1007/s11771-007-0047-7 ![]()
Erosion characteristic of slope sandstone soaking in acid mine drainage
JIANG Li-chun(姜立春)1, CHEN Jia-sheng(陈嘉生)2, WU Ai-xiang(吴爱祥)2
(1. Institute of Safety Engineering, South China University of Technology, Guangzhou 510640, China;
2. School of Resources and Safety Engineering, Central South University, Changsha 410083, China)
Abstract:
Acid mine drainage(AMD) is one of the main reasons of slope instability in chemical mines with high sulfide. The pH values of the solution inside the mining pit decrease with the increasing of distance from ore body and vary from 1.2 to 4.6, according to the results of the water environmental investigation and the composition test of the slope sandstone in Xinqiao Pyrite Mine. Comparative experiments between original sandstone and AMD eroded sandstone samples show that after AMD erosion the uniaxial compressive strength and elastic modulus decrease by 30%-50% and 25%-45%, respectively, the cohesion and internal friction angle decrease obviously, and the Poisson ratio fluctuates between 0.20-0.29. The greater joints development, the higher residual stress after peak value, and the longer time to damage. Besides above, the reaction mechanism analysis of AMD eroded sandstone shows that the fillings in joints and fissures of sandstone are frequently decomposed and polyreacted, resulting in changes of interior molecule structure and framework composition, and decreases of cohesion and angle of internal friction between rock structure interfaces.
Key words:
acid mine drainage; slope; microstructure; sandstone ;
1 Introduction
The international researches on the damage of slope rock by water are major focused on osmosis damage for a long period, but damage by acid mine drainage(AMD) has not been drawn high attention[1-4]. In fact, AMD transforms microstructure of rock, permutes and dissolves composition, enlarges structural interface brings out new fissures. Long term AMD erosion slope rock would decrease the mechanics property of a part of rocks. The slope rocks in special environment such as chemical (mental) mines and coal mines have abundant sulfide minerals, they react with water and air, and generate acid, which is easy to react with slope rock as limestone and sandstone changing interior molecule structure and framework composition, decreasing cohesion and angle of internal friction between rock structure interfaces. The fissures in the exposure rock mass continually extend along with the development of excavation, and it would lead to collapse of part slope and even landslide.
International scholars have relevant researches in this field[1,5-15]. VANDIVIERE et al[5] studied AMD erosion mechanism of limestone, phosphate and silica on ore piles based on outdoor and indoor experiments. NYAVOR et al[6] analyzed the technical measures to prevent AMD erosion in Canada coal mine. GU[7] studied the influence of acid and alkali solutions on engineering mechanics property of foundation soil, as well as the chemical denudation and the long-term strength and stability problem of the cemented medium in soil mass. WANG et al[8] researched the characters of rock breaking and fractal geometry under the chemical erosion condition. TANG et al[9] carried out exploratory research on mechanics effect on silihydrite and calcite by water-rock chemical attack. LI et al[10] studied the influence of AMD on rock elastic modulus and uniaxial compressive strength. In general, the scholars have not yet systematic understanding the micromechanics effect of the rock erosion by AMD erosion, few of related references with engineering example have been reported, and relevant mechanism researches need further development. This article is about the research on how AMD influences mechanics properties and structure of the slope, based on the investigation of the slope environment in Xinqiao deep-concave open pit, indoor AMD simulation experiment and microstructure analysis. It aims at offering technology support for the slope harness and prevention of the geologic engineering disaster.
2 Engineering background
Xinqiao Pyrite Mine is a deep-concave opencast, its major ore is pyrite, the slope height of design is 426--180 m and it is now excavated to -68 m, the average annual rainfall is about 1 330 mm. The current water drainage system cannot meet the requirement of production schedule, and part rainfall is cumulated at the top of orebody along with the mining extension. With the disturbing by explosive wave and mechanical vibrating, the exposed original close texture of pyrite body has been damaged, ore lump size becomes small, and the contact area between atmosphere and water increases, the pyrite continually oxidizes, bringing out a mass of acid solution that ceaselessly reacts with Gaolishan sandstone and shale of the slope, changing rock microstructure and decreasing macroscopic strength of the slope, all above lead to the large area collapse of slope and local instability problem each year, it has brought about great economic loss to the mine. Fig.1 shows the exterior of serious erosion at the slope bottom. Fig.2 shows the limestone grains soaked by AMD.

Fig.1 Erosion exterior of slope

Fig.2 Limestone grains soaked by AMD
2.1 Rock compositions of slope
Excavation reveals that the slope in Xinqiao open-pit is mostly made of quartz sandstone, arenaceous shale, limestone, feldspar and siliceous rock, major ore compositions are quartz, diorite, calcite, and anorthite, etc. There are a mass of mudstone between faults and joints, major components are illite, chlorite and kaolinite, etc. Parts of them are with few of calcite, limonite and zeolite, etc. These materials tend to degenerate when they encounter acid, cement material can be decomposed and educed, which decreases the friction between rock layers.
2.2 Solution ingredient of slope
Solution samples were taken out from the seepage water on the slope surface, the waterhouse at bottom of stope, the sump and the stream outside of mining pit, on the 11#-21# exploratory line above -48 m horizon, total 26 samples. Test results show (Table 1) the solution with acidity in the mining pit, pH value is between 1.2 and 4.6. Solution of seepage field is also with acidity, the closer to mining body, the smaller pH value. Generally, the solution of the circumference outside the mining pit is neutrality.
Table 1 Composition analysis results of part samples
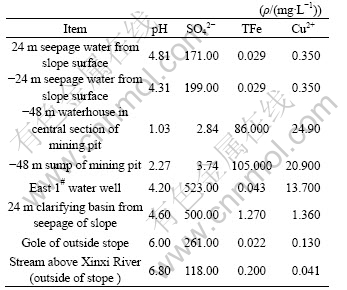
3 AMD simulation experiment
In order to further study the erosion mechanism of AMD to the slope rock, the fresh sandstones were taken out from the slope in Xinqiao open-pit and processed to standard rock specimens. According to the analysis results of water-rock environment of the slope, sulphuric acid solutions with the same concentration were applied for specimens dipping. Under the condition of room temperature and pH value without change, the samples were separately taken out at the same time steps of 5 d, then the compression test and tensile test were carried out after atmospheric drying (holding original water content), and a variety of rock mechanic parameters were observed including shear strength, compression strength, elastic modulus and the Poisson ratio.
3.1 Testing material
3.1.1 Samples
The samples were sandstones from the middle section (12 m level) of the slope between 17# and 19# exploratory line. For the convenience of comparative analysis, all samples were from the same raw material, and processed strictly in accordance with criterion, the samples with disfigurement were excluded. The sandstone samples contained lots of fissure and fillings after processing. The fillings were cemented iron substrate, calcium substrate and mud substrate in the form of pore-contact cementation, and the fissures distributed in multiway with complex shape. The joints and fillings have been marked, recorded and taken photos before test.
For the sake of comparation, the samples were separated into 4 groups, 6 pieces in each group, total 32 pieces based on joints development and fillings difference. Thereinto: samples with better developed joint fissure in groups 1 and 2, samples with developed cemented fillings in groups 3 and 4. Samples for tensile test were in 2 groups, 9 pieces in each group, and 18 pieces in total. Thereinto: samples with developed joint fissure were in group 1, samples with developed cemented fillings were in group 2.
3.1.2 Acid solution
Based on the solution analysis results in mining pit, H2SO4 solution, at pH values of 1.07 and 3.26 were confected. Compression test samples: sandstone group 1 (fissure) and group 3 (fillings) were put into the solution at pH 1.07, group 2 (fissure) and group 4 (fillings) were dipped into the solution at pH 3.26; tensile test samples were separately put into the two tanks with different solutions. 1-2 test pieces were taken out from each solution at the time intervals of 5 d for uniaxial compression test and tensile test, and then compared with the result of non-soaking pieces. The room temperature was kept at 20 ℃(±5 ℃) during the 25 d of experiment.
3.1.3 Experiment system
Mechanical property tester for rock was INSTRON-1342 material testing machine with electrohydraulic servo control, ultimate load was 250 kN, rate of loading was 3.75 nm/s, control application software was wave marker special for electrohydraulic servo.
3.2 Compressive experiment
Based on the process result of non-soaking samples during loading, the surface of rock presented visible cracks paralleling to compressing stress direction when the load was up to 60%-70% of peak strength of rock. The crack spread along with the compressing stress direction with increasing loading followed the slabbing around the specimen. The destructive process was accompanied with energy releasing, great sound came up at the end of loading, there were small pats coming down. The failure mechanism was patulous abruption as well as attrition crushing.
Sandstone samples after dipping for 5 d were loaded and then gradually deformed, visible crack came up after a while, the cracks were still parallel to the compressing stress direction, it was ended with tensional fracture damage dilating at the middle of rock samples, and the sound was smaller than previous one. With increasing soaking time, abrupt discharge of damage strain energy was gradually weakened. The test process showed that the damage time from crack coming up to the eventual failure was getting longer with increasing soaking time; after the peak value appeared, the samples were still with a great residual stress, descent speed gradient of failure strain was weakened. For the non-soaking samples, the time from the crack emergence to the eventual failure was instant, after achieved the peak value, the loading stress decreased rapidly. The stress—strain curves of samples are shown in Fig.3.
Joints and fillings have big impact on the samples intensity, from Fig.3, stress concentration comes up at the fillings-sample interface during loading, the fillings drops out, stress amplitude of loading has great fluctuation, rebounds at part area. And the more fillings in the samples, and the larger rebounding amplitude of loading stress, the more loading times, the larger area to drop out fillings. The Poisson ratio of sandstone with high percentage of crack and fillings is not a fixed value any more, it fluctuates within a range. Poisson ratio of sandstone samples is between 0.20-0.29 according to result of calculation.
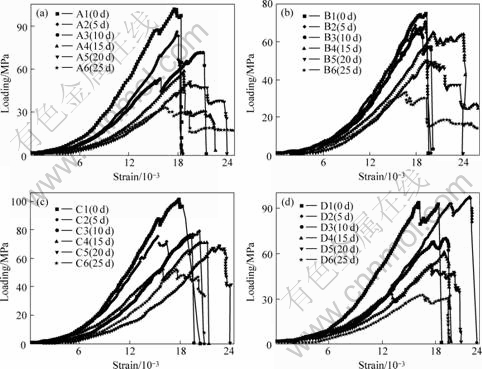
Fig.3 Uniaxial compressive stress—strain curves of sandstone with fissures and joints
(a) Group A of sandstone (cemented fillings, pH=1.07); (b) Group B of sandstone (crack, pH=1.07);
(c) Group C of sandstone (fillings, pH=3.26); (d) Group D of sandstone (crack, pH=3.26)
The cracks are frequently splayed and combined during the loading process based on the radial crack developing curve of sandstone samples (Fig.4). The larger ultimate load, the higher joints development, and the bigger the residual stress, the longer damaging time after the peak value came up.
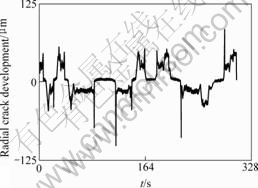
Fig.4 Radial crack development—time curve of sandstone radial crack
n the same soaking time, pH values of solution have impacted on the sample deformation. The smaller pH value, the bigger crack distribution density once failed, and the more uniform, the bigger area. On the contrary, the smaller density, the more no uniformity, and the smaller crack area.
Test results indicate that compression strength of rock continues to decrease along with increase of soaking time for samples, the value decreases by 30%-50% (Fig.5), and elastic modulus decreases by 25%-45% (Fig.6). Fissures and joints inside rock speed up the AMD progress, dissoluble fillings are the major objects of erosion.
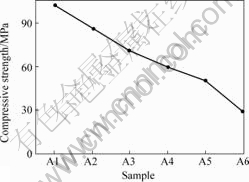
Fig.5 Compressive strength vs soaking time curve of group A of sandstone(A1: 0 d; A2: 5 d; A3: 10 d; A4: 15 d; A5: 20 d; A6: 25 d)

Fig.6 Elastic modulus of sample of group A(A1: 0 d; A2: 5 d; A3: 10 d; A4: 15 d; A5: 20 d; A6: 25 d)
3.3 Tensile experiment
The tensile experiment results of sandstone with soaking were complicated. In general, the tensile strength of samples decreases along with longer soaking time, after 15-25 d, it decreases by 30%-50% at least (Fig.7), and the elastic modulus becomes small. This implies that the acid solution has strong erosion effect on the sandstone, changing the internal media composition of rock, part of them can be replaced by dissoluble ion, framework becomes loose, and the entire tensile strength and elastic modulus are decreased.

Fig.7 Tensile strength of sandstone soaked at different time (pH=1.07)
3.4 Microstructure
In order to further study the microstructure of samples before and after reaction, the sandstone samples before and after soaking in water were observed by 100 multiple microscope. Fig.8 shows the surface topography observation results of samples before and after soaking. From the area with arrow, it can be seen that the microstructure of sample surface changes with the increase of soaking time, the iron-substrate between joints is eroded and dissolved, and the metallic ion is migrated.

Fig.8 Microstructures of samples before and after soaking in water(a) Before soaking; (b) After soaking
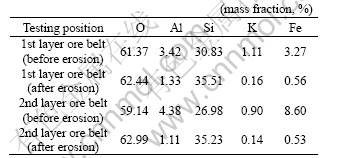
In order to further understand the microstructure of samples by AMD, the samples were scanned by SEM (scanning electron microscope) (JSM-6301F) under the 1 500 magnifications condition, the results are shown in Figs.9-11. Fig.9 and Fig.10 show the images before and after erosion by AMD, which proves that slight amounts of ores between joints are dissolved. Fig.11 indicates that Fe and Al elements are dissolved and diluted after AMD erosion, only Si element is residue that is difficult to dissolve. Table 3 shows the element mass fraction of samples before and after AMD erosion, it can be seen that acid solution has strong erosion effect on the crystal inside of rock, mass fractions of Al, Fe element are greatly decreased after AMD.

Fig.9 SEM images of samples eroded by AMD(a) Before reaction; (b) After reaction

Fig.10 Elements distribution of samples eroded by AMD

Fig.11 Element spectrograms of sample eroded by AMDBefore reaction; (b) After reaction
Table 3 Element composition of sample eroded by AMD
4 Reaction mechanism
There is a mass of sulfide minerals in the chemical mine, pit of mental ore, ore piles and waste piles. They are exposed to the air, and continue to react with the oxygen and water, bringing out acid liquid, which keeps on oxidizing with surrounding medium, for example, FeS2. Its chemical reactions are follows.
1) Pyrite is oxidized with water and oxygen,generating ferrous sulfate and sulfuric acid.
2FeS2+7O2+2H2O→2FeSO4+2H2SO4 (1)
2) Ferrous sulfate continues to be oxidized to ferric sulfate. (Chemical oxygen would be completely stopped in acid environment).
4FeSO4+O2+2H2SO4→2Fe2(SO4)3+2H2SO4 (2)
The erosion reaction cannot take place in acid environment, the ferric sulfate would be deoxidized to ferrous sulphate by pyrite at low pH value.
3) Ferric sulfate reacts with pyrite, then
e2(SO4)3+FeS2→3FeSO4+2S (3)
2S+6Fe2(SO4)3+8H2O→12FeSO4+8H2SO4 (4)
4) Part of ferri sulfate is hydrolyzed to alkaline ferric sulfate.
Fe2(SO4)3+2H2O→2Fe(OH) SO4+H2SO4 (5)
The sulfuric acid was washed away by running water or dissolved in the water and increased acidity of water. At the same time, there were copper sulphate and ferrous sulphate in the oxidized mineral, they would react with the acid solution and bring out new sulfuric acid.
FeS2+14CuSO4+12H2O→
7Cu2S+5FeSO4+12FeSO4 (6)
2CuSO4+2FeSO4+2H2O→Cu2O+Fe2(SO4)3+H2SO4 (7)
FeS2+14Fe3++8H2O→15Fe2++2SO42-+16H+ (8)
The chemical actions of mineral in the acid environment can be separated into two types: mineral decomposition and polyreaction.
1) The chemical reactions of decomposition are shown as follows:
Mg2Si2O4+4H+→2Mg2++H4SiO4 (9)
CaMgSi2O6+12H+→Ca2++Mg2++2H4SiO4 (10)
CaAl2Si2O6+2CO2+3H2O→Al2Si2O5(OH)4+
Ca2++2HCO3-
(11)
Mg5Al2Si3O10(OH)8+16H+→5Mg2++2Al3++
3H2SiO4+H2O
(12)
CaCO3+2H++SO42-+H2O→CaSO4?2H2O+CO2 (13)
MgCO3+2H++SO42-+6H2O→MgSO4?7H2O+CO2 (14)
Al2Si2O5(OH)4+6H+→2Al3++2H4Si4O(aq)+2H2O (15)
2) Major poly-reactions in the acid medium are shown as follows:
Fe2++SO42-+7H2O→FeSO2?7H2O (16)
Mg2++SO42-+7H2O→MgSO4?7H2O (17)
2Al3++2H4Si4O+2H2O→Al2Si2O5(OH)4+6H+ (18)
There are great deal of detrital rock, clay mineral, siliceous rock and carbonate rock inside the faultages, fissures and joints of the slope in Xingqiao pyrite mine, the physical mechanic properties of these fillings would have significant change in the acid environment. The major mineral of detrital rock is quartz, it can be regarded as inert mineral without reaction, the quartz grains act as underprop in the rock texture. Feldspar, muscovite, etc act as underprop and interstitial fillings in the rock texture. But the feldspar and muscovite would gradually lose alkali metal ions such as K+ and Na+ in the acid environment, and transform to kaolin mineral. Mudstone is made of clay mineral, and its major composition is illite, and next chlorite and kaolinite, etc. Clay mineral in medium is with poor stability in acid environment, it is easy to metatropy and decompose. There are few calcite, limonite and zeolite in mudstone, in general these minerals play the role in the cementation of the rock texture, and the cementation would be weakened in the acid environment.
Siliceous rock is mostly made of quartz, cryptocrystalline silicon dioxide and amorphous silica. The amorphous silica has chemical activity in the acid medium, there is polyreaction with aluminium ion in the solution on the occasion of pH=1-5, generating the kaolinite mineral. Siliceous rock contains slight amount or minute clay and feldspar mineral, which generally act as filled opening, the chemical behavior in acid medium would influence the rock strength. The major compositions of carbonate rock are calcite, dolomite, slight amount of mesitite and siderite, and other kinds of carbonate minerals, these minerals have acute decomposition reaction in the acid medium.
5 Conclusions
1) Based on the water environment investigation of mining pit and analysis in the Xinqiao Pyrite Mine, the pH values of solution in the mining pit distribute between 1.2-4.6, the closer to mine body, the smaller pH value.
2) Under the conditions of different time steps, AMD simulation experiment for eroding sandstone by two kinds of H2SO4 at pH values of 1.07 and 3.26 indicates that uniaxial compressive strength of eroded sandstone decreases by 30%-50%, elastic modulus decreases by 25%-45%, cohesion and angle of internal friction also fall off, the Poisson ratio fluctuates between 0.20 and 0.29, the radial cracks are frequently splayed and combined during the loading process. The higher joints development, the bigger residual stress after achieving peak value, and the longer damage time.
3) Some composition elements of sandstone (such as Fe, Al) are dissolved and diluted after AMD, mass fractions of the elements greatly descend.
4) Mechanism analysis indicates decomposition and polyreaction of the fillings of joints and fissures in the sandstone frequently occur during AMD erosion, interior molecule structure and framework composition are altered, cohesion and angle of internal friction between bedded planes of rock mass are decreased.
References
[1] JIANG Li-chun. Study on Safety Environment of High-Deep Concave Slope in Open Pit & Engineering Control[D].Changsha: Central South University, 2005.(in Chinese)
[2] SUN Guang-zhong. Typical Landslide in China[M]. Beijing: Science Press, 1998.(in Chinese)
[3] YANG Zhi-fa, DING En-bao, SUN Yu-ke. Slope Stability Research in Chinese Open Mining[M]. Beijing: China Science and Technology Press, 1998. (in Chinese)
[4] WANG Gong-xian. Key technique in landslide control and its handling measures[J]. Chinese Journal of Rock Mechanics and Engineering, 2005, 24(21): 3818-27.(in Chinese)
[5] VANDIVIERE M M, EVANGELOU V P. Comparative testing between conventional and microencapsulation approaches in controlling pyrite oxidation[J]. Journal of Geochemical Exploration, 1998, 64: 161-176.
[6] NYAVOR K, EGIEBOR N O. Control of pyrite oxidation by phosphate coating[J]. The Science of the Total Environment, 1995, 162: 225-237.
[7] GU Ji-wei. Engineering quality attacked by acid-alkali waste solution influence on foundation soil[J]. Chinese Journal of Geotechnical Engineering, 1988, 10(4): 72-78.(in Chinese)
[8] WANG Yong-jia, FENG Xia-ting. Micro-fracture properties of rock affected by chemical environments(PartⅡ): Temporal fractal analysis[J]. Chinese Journal of Rock Mechanics and Engineering, 2000, 19(5): 551-556.(in Chinese)
[9] TANG Lian-sheng, ZHANG Peng-cheng, WANG Si-jing. Testing study on effects of chemical action of aqueous solution on crack propagation in rock[J]. Chinese Journal of Rock Mechanics and Engineering, 2002, 21(6): 822-827.
[10] LI Ning, ZHU Yun-ming, ZHANG ping. A chemical damage model of a sandstone in acid environment[J].Chinese Journal of Geotechnical Engineering, 2003, 25(4): 395-399.(in Chinese)
[11] SRACEK O, CHOQUETTE M, GELINAS P, et al. Geochemical characterization of acid mine drainage from a waste rock pile[J]. Journal of Contaminant Hydrology, 2005, 69: 45-71.
[12] MATSUKURA Y. The role of the degree of weathering and groundwater fluctuation in landslide movement in a colluviums of weathered hornblende-gabbro[J]. Catena, 1996(27): 63-78.
[13] HAMMARSTROM J M. Characterization of limestone reacted with acid-mine drainage in a pulsed limestone bed treatment system at the Friendship Hill National Historical Site, Pennsylvania, USA[J]. Applied Geochemistry, 2003, 18: 1705-1721.
[14] BENZAAZOUA M, MARION P, PICQUET, BUSSIERE B.The use of paste fill as a solidification and stabilization process for the control of acid mine drainage[J]. Minerals Engineering, 2004, 17: 233-243.
[15] FARAH A, HMIDI N, MOSKALYK R, et al. Numerical modeling of the effectiveness of sealants in retarding acid mine drainage from mine waste rock[J]. Canadian Institute of Mining and Metallurgy, 1997, 36(4): 241-250.
Foundation item: Project(50321402) supported by the National Science Fund for Innovative Research Group; project(2004CB619206) supported by the Major State Basic Research Development Program of China; project (50325414) supported by the National Science Fund for Distinguished Young Scholars
Received date: 2006-02-24; Accepted date: 2006-07-27
Corresponding author: JIANG Li-chun, PhD; Tel:+86-20-87114740; E-mail:gingerjlc@126.com
(Edited by LI Xiang-qun)
Abstract: Acid mine drainage(AMD) is one of the main reasons of slope instability in chemical mines with high sulfide. The pH values of the solution inside the mining pit decrease with the increasing of distance from ore body and vary from 1.2 to 4.6, according to the results of the water environmental investigation and the composition test of the slope sandstone in Xinqiao Pyrite Mine. Comparative experiments between original sandstone and AMD eroded sandstone samples show that after AMD erosion the uniaxial compressive strength and elastic modulus decrease by 30%-50% and 25%-45%, respectively, the cohesion and internal friction angle decrease obviously, and the Poisson ratio fluctuates between 0.20-0.29. The greater joints development, the higher residual stress after peak value, and the longer time to damage. Besides above, the reaction mechanism analysis of AMD eroded sandstone shows that the fillings in joints and fissures of sandstone are frequently decomposed and polyreacted, resulting in changes of interior molecule structure and framework composition, and decreases of cohesion and angle of internal friction between rock structure interfaces.


