
Extraction and purification of magnetic nanoparticles from
strain of Leptospirillum ferriphilum
GAO Jian(高 健)1, 2, XIE Jian-ping(谢建平)1, DING Jian-nan(丁建南)1,
KANG Jian(康 健)1, 2, CHENG Hai-na(程海娜)1, QIU Guan-zhou(邱冠周)1
1. School of Resources Processing and Bioengineering, Central South University, Changsha 410083, China;
2. School of Life Science, Hunan University of Science and Technology, Xiangtan 410201, China
Received 20 January 2006; accepted 24 April 2006
Abstract:
The magnetic nanoparticles were extracted from Leptospirillum ferriphilum, strain YSK, isolated from acid mine drainages by treatment with sodium dodecyl sulfate(SDS) and centrifugation through a sucrose density gradient. Transmission electron microscopy(TEM) indicates that the nanoparticles are approximately spherical with a mean diameter of 44 nm, and magnetite crystals in this size range are single magnetic domains. Energy-dispersive X-ray analysis shows that the nanoparticles primarily contain two kinds of elements, iron and oxygen. Thus it can be concluded that the magnetic particles are magnetosomes. Generally, it is thought that cellular magnetotaxis is a direct consequence of the cell possessing magnetosomes. The discovery of magnetosomes in strain YSK can provide the theoretical basis for screening efficient bioleaching bacteria which are specific to different magnetic minerals under an outer magnetic field.
Key words:
Leptospirillum ferriphilum; strain YSK; SDS; sucrose density gradient; magnetosomes; magnetotaxis;
1 Introduction
From 1975, there is much interest in finding magne- totactic bacteria[1], because bacterial magnetosome particles, unlike those produced chemically, have a consistent shape, a unique crystallography, a narrow size distribution within the single magnetic domain range and so on. Therefore, there may be a new path for synthesizing particular magnetic nanomaterials by magnetotactic bacteria[2]. MATSUNAGA[3] predicted that magnetosomes synthesized by magnetotactic bacteria would be a novel bioresource for application in high technology. Presently, magnetosomes have been used in magnetic domain analysis[4], wastewater recovery[5], detection of environmental microorganisms, and other commercial applications including the development of novel materials[6,7], recognition of RNA, extraction and detection of DNA[8,9], the diagnosis of tumour and so forth. Moreover, it is worthwhile to mention that the magnetic iron-storage protein, magnetoferrtin, has shown numerous applications in biological and medical technology[10].
Magnetotactic bacteria are cosmopolitan in distribution and ubiquitous in aquatic habitats with few exceptions, but they have not been found in significant numbers in well aerated[11] or in acidic aquatic environments such as acid mine drainages(AMD). The extraction of magnetosomes from the genus leptospirillum has not been reported. On the basis of similarities between the strain YSK and magnetotactic bacteria reported (Table 1), we speculated that the extremely acid environment is likely to benefit the formation of magnetosomes. The major objectives of this work are to attempt to extract magnetosomes from the strain YSK and provide the theoretic basis for screening efficient bioleaching bacteria which are special to different magnetic minerals under outer magnetic field.
2 ExperimentalThe strain YSK (16S rDNA sequence GenBank access number, DQ343299) was cultured in growth medium containing the following components (g/L): (NH4)2SO4 3.00; KCl 0.10; K2HPO4 0.50; MgSO4·7H2O 0.50; Ca(NO3)2 0.01; FeSO4·7H2O 44.2. The pH was adjusted to 1.6 with 5 mol/L H2SO4. Incubation was shaken at 180 r/min, 40 ℃ after the addition of 1% (volume fraction) inoculum.
Table 1 Comparison of properties between strain YSK and magnetotactic bacteria
Cells concentrated by centrifugation from the growth medium were washed three times with 0.02 mol/L H2SO4. Cells were then resuspended in PBS buffer, and the cell suspensions were centrifuged for 5 min at 12 000 r/min and the supernatant was discarded. The above process was repeated several times until the supernatant did not exhibit any further color change (red) when several drops of H2O2 and 0.5 mL of 0.2% KSCN were added respectively.
After dehydration with 95% ethyl alcohol, Fe-free cells were fixed by adding 1% OsO4 to the culture medium to bring the final OsO4 concentration to 0.1%. Cells were then concentrated by centrifugation, washed in Kellenberger buffer, suspended in tryptone-salt solution, and embedded in low viscosity, thermally curing Epon resin. Ultrathin sections were cut from the resin blocks by using a Leica Microsystems. The sections were transferred to 50 μm formvar Cu TEM grids for image analysis.
Approximately 0.5 g of Fe-free cells was added to a 2 mL Eppendorftube containing 1.0 mL TE buffer (pH=8.0) and 100 μL of 20%SDS. The mixture was agitated gently by vortexing for 30 s and incubated at 55 ℃ for 10-30 min. The magnetic particles were then immobilized on a PromegaTM magnetic stand and the supernatant was discarded. After washing twice with PBS buffer, the magnetic particles were again washed twice with anhydrous ethanol. After the evaporation of ethanol at room temperature, the magnetic particles were purified and collected by 40%-80% sucrose gradient centrifugation (6 000 r/min, 10-20 min) according to XIE et al[12]. The purified pellets were frozen dry by vacuum and then placed on grids directly.
Samples were observed and recorded with a JEOL JEM-1230 TEM at an accelerating voltage of 80 kV. Scanning electron microscopy(SEM) and energy- dispersive X-ray analysis were performed at 20 kV and 80 kV respectively using a JEOL JSM-6360 LV scanning electron microscope equipped with an EDX-GENESIS60S X-ray analyzer.
3 Results and discussion
3.1 Cell morphology
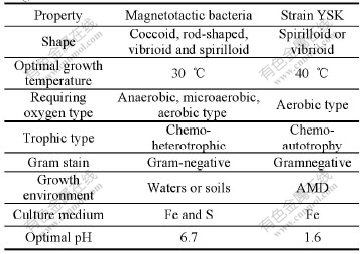
Cells of strain YSK are small curved rods or spirilla (Fig.1). Critical-point-dried cells have relatively constant diameter (0.278-0.421 μm; average 0.35 μm), but their lengths vary considerably from 1.54 μm to 3.17 μm (average 2.36 μm). The variation results from the fact that young cells are vibrio shaped, but in cultures older than 5 d, cells are mostly spiral shaped with two to four turns of the helix, even though approximately 60% of the examined cells consist of two turns or less.
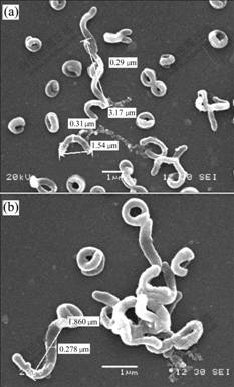
Fig.1 SEM images of cell preparations of strain YSK
3.2 TEM of thin-sectioned preparations
TEM of thin-sectioned preparations shows that cells of strain YSK contain only 3-5 electron-dense particles that are readily observed(Fig.2), but approximately 30% of the cells lack the particles entirely. The largest particles are situated in the center or bottom of the cell. The particles were intracellular, i.e., they are located inside the cytoplasmic membrane. The electron-dense particles in the cells of strain YSK are not arrange in a single chain. This result is similar to that reported by RODGERS et al[13].
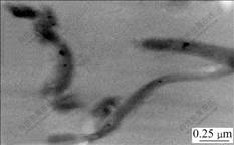
Fig.2 TEM morphology of ultrathin section of cells of strain YSK
3.3 TEM and energy-dispersive X-ray analysis of magnetic particles
Transmission electron micrograph of the purified particles (Fig.3) indicates that these particles in the cells of strain YSK are round-like or cuboidal in shape and are 24-64 nm (average 44 nm) in diameter. Energy- dispersive X-ray analysis (Fig.4) indicates that the magnetic particles in the cells of strain YSK mostly consist of two kinds of elements, iron and oxygen, of which the element iron accounts for 50.15% and oxygen for 26.38%. Basic analysis shows that the mineral phase of the magnetic particles is composed of Fe3O4. The detected carbon (Fig.4) can be attributed to electron- dense layer surrounding magnetic particles.
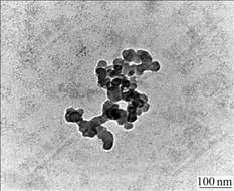
Fig.3 TEM morphology of magnetic particles purified
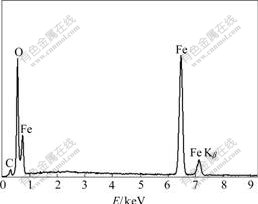
Fig.4 Energy-dispersive X-ray analysis of magnetic particles
To date, the cellular morphologies of magnetotactic bacteria reported include coccoid, rod-shaped, vibrioid and spirilloid. Strain YSK, isolated from acid mine drainages(AMD) of Dexing Copper Ore of China, belongs to the genus Leptospirillum; Gram-negative members of the domain Bacteria; vibrioid and spirilloid. Therefore, in morphology, strain YSK is in accordance with basic properties of magnetotactic bacteria reported previously. On the other hand, the mineral phases of the magnetosomes reported are of two general types, iron oxides and iron sulfides[14]. Iron oxide-type magnetosomes contain particles of ferromagnetic mineral magnetite (Fe3O4), while the iron sulfide-type contains ferromagnetic greigite (Fe3S4), greigite and non- magnetic pyrite (FeS2), or possibly ferromagnetic pyrrhotite (Fe7O8). Regardless of their compositions, the crystalline particles in magnetosomes have a narrow size range: approximately 25-120 nm. Magnetite crystals within this size range are single magnetic-domains. In this work, the size (average 44 nm) of the particles formed by strain YSK is just within the size of the calculated stability field of single-domain magnetite. Furthermore, the strain YSK, when subjecting to an outer magnetic field, shows weak magnetotaxis. Generally, it is thought that cellular magnetotaxis is a direct consequence of the cell possessing magnetosomes[14]. Thus it can be concluded that these particles in cells of strain YSK are magnetosomes.
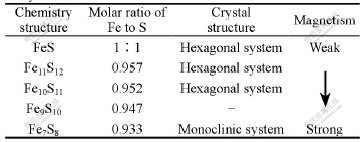
It was reported[15] that the magnetism of Pyrrhotite can change with ratio of Fe to S (Table 2). The isolates from a medium containing Pyrrhotite with a relatively weak magnetism usually possess magnetic particles. The bacteria have a higher Fe2+-oxidized efficiency in the process of pyrite bioleaching. Therefore, the discovery of the weak magnetotaxis of strain YSK can provide the theoretic basis for screening efficient bioleaching bacteria which are special to different magnetic minerals under an outer magnetic field.
Table 2 Comparison between crystal structure and magnetism of Pyrrhotite
Though in most magnetotactic bacteria the magnetosomes are arranged in one or more chains[16, 17], the magnetosomes of strain YSK are not arranged in chains in this study. Of the cells observed, about 70% contains only 3-5 particles, which is possibly due to the difference between the genetic characteristics and growing condition of strain YSK. Generally, the magnetite-produced magnetotactic bacteria prefer the OATZ and behave as oxygen-respiring microaerophiles [18]. So far, about 20 species of magnetotactic bacteria are found, among which several strains of aerobic magnetotactic bacteria, i.e. AMB, HM-1 and YSC-1, are isolated from seawater and freshwater[19-21], but most are heterotrophic, anaerobic and difficult to attain pure culture[22]. Difficulties in cultivating magnetotactic bacteria result from their growth pattern, which is adapted to sediments and chemically stratified aquatic habitats [23]. More efforts are needed in searching suitable conditions for laboratory mass cultivation. However, strain YSK, which belongs to chemolithoautotrophic and aerobic bacteria, is isolated from extremely acidic environments and easily cultured. Therefore, the aerobic magnetotactic bacterium strain YSK supplies a reference material for biomagnetism and bionics. On the other hand, the largest number of magnetotactic bacteria reported previously is found at or just below the oxic-anoxic boundary or transition zone(OATZ). The discovery of the aerobic strain YSK also challenges the OATZ theory. Furthermore, with uniform granularity, high specific surface area and avirulence to cell, magnetosomes will bring about countless potential value in many fields. So, recent progress in techniques for isolating and culturing strain YSK offers possibility for the large-scale production of bacterial magnets.
4 Conclusions
1) The magnetic particles are isolated from strain YSK by treatment with sodium dodecyl sulfate and centrifugation through a sucrose density gradient.
2) Transmission electron microscopy and the energy profile analysis indicate that the magnetic particles are magnetosomes.
3) The existence of magnetosomes in strain YSK is in accordance with its weak magnetotaxis exhibited under an outer magnetic field.
References[1] BLAKEMORE R.P. Magnetotactic bacteria [J]. Science, 1975, 190: 377-379.
[2] SARIKAYA M. Biominetics: materials fabrication through biology [J]. Proc Natl Acad Sci USA, 1999, 96(25): 14183-14185.
[3] MATSUNAGA T. Applications of bacterial magnets [J]. Trends Biotechnol, 1991, 9(3): 91-95.
[4] FUTSCHIK K, PFUETZNER H, DOBLANDER A, DOBENECK T, PETERSEN N, VALIH. Why not use magnetotactic bacteria for domain analyses?[J]. Physica Scripta, 1989, 40: 518-521.
[5] BAHAJ A S, CROUDACE I W, JAMES P A B, MOESCHLER F D, WARWICK P E. Continous radionuclide recovery from wastewater using magnetotatic bacteria [J]. J Magnet Magnet Mater, 1998, 184: 241-224.
[6] MANN S. Biomineralization: Principles and Concepts in Bioinorganic Materials Chemistry [M]. Oxford: Oxford University Press, 2001. 6-23.
[7] CAO X B, XIE Y, YU F, YAO Z Y, LI L Y. Magnetic force driven orientation of Co23B10 array inspired by magnetotactic bacteria [J]. J Mater Chem, 2003, 13: 893-896.
[8] YOZA B, MATSUMOTO M, MATSUNAGA T. DNA extraction using modified bacterial magnetic particles in the presence of amino silane compound [J]. J Biotech, 2002, 94: 217-224.
[9] MATSUNAGA T, NAKAYAMA H, OKOCHI M, TAKEYAMA H. Fliorescent detection of cyanobaterial DNA using bacterial magnetic particles on a MAG-microarray[J]. Biotech Bioengi, 2001, 73: 400-405.
[10] YAMAZAKI G, URAOKA Y, FUYUKI T, YAMASHITA I. Nano-etching using nanodots mask fabricated by bio-nano-process[J]. J Photopoly Sci Techno, 2003, 16: 439-444.
[11] BLAKEMORE R.P. Magnetotactic bacteria [J]. Ann Rev Microbiol, 1982, 36: 217-238.
[12] XIE Jian-ping, LIU Xin-xing, LIU Wen-bin, QIU Guan-zhou. Extraction of magnetosomes from a strain of Acidithiobacillus ferrooxidans [J]. Biomagnetism, 2005, 5(3): 7-10.
[13] RODGERS F G,BLAKEMORE R P, BLAKEMORE N A, FRANKEL R B, BAZYLINSKI D A, MARATEA D, RODGERS C. Intercellular structure in a many-celled magnetotactic prokaryote [J]. Archives of Microbiology, 1990, 154(1): 18-22.
[14] BAZYLINSKI D A. Synthesis of the bacterial magnetosome: the making of a magnetic personality [J]. Internal Microbiology, 1999, 2: 71-80.
[15] LIU Xin-xing, XIE Jian-ping, HUO Qiang, QIU Guan-zhou. New method of strain isolation by magnetic separation—strain isolation by using pyrrhotite culture medium [J]. Metal Mine, 2005, 354(12): 26-29.
[16] BAZYLINSKI D A. Structure and function of the bacterial magnetosome [J]. ASM News, 1995, 61: 337-345.
[17] BAZYLINSKI D A, MOSKOWITZ B M. Microbial biomineralization of magnetic iron minerals: microbiology, magnetism and environmental significance [J]. Rev Mineral, 1997, 35: 181-223.
[18] BUSECK P R, DUNIN-BORKOWSKI R E, DEVOUARD B, FRANKEL R B, McCARTNEY M R, MIDGLEY P A, P?SFAI M, WEYLAND M. Magnetite morphology and life on Mars [J]. PNAS, 2001, 98(24): 13490-13495.
[19] MATSUNAGA T, SAKAGUCHI T, TADOKORO F. Magnetite formation by a magnetic bacterium capable of growing aerobically [J]. Applied and Microbiology Biotechnology, 1991, 35: 651-655.
[20] ZHANG Yong-liang, WEI Yang-hao, YANG Qing-xian. Isolation of a aerobic magnetotactic bacterium HM-1 and studies on its biological charactersltics [J]. J Wuhan Univ (Natural Science Edition), 1997, 43(6): 775-779.
[21] GAO Jun, XIAO Tian, SUN Song, CHEN Guan-jun, ZHAO Yong, WU Long-fei. Isolation of a novel marine magnetotactic bacteria YSC-1 and studies on the highly uniform, magnetic nano-material magnetosome [J]. High Tech Letters, 2004, 5: 44-47.
[22] BAZYLINSKI D A, FRANKEL R B. Magentosome formation in prokaryotes [J]. Nature Review, 2004, 2: 217-230.
[23] SCH?LER D, FRANKEL R B. Bacterial magnetosomes: microbiology, biomineralization and biotechnological applications [J]. Appl Micro-Biol Biotechnol, 1999, 52: 464-473.
Foundation item: Project(2004CB619201) supported by the National Basic Research and Development Program of China; Project(50321402) supported by the National Natural Science Foundation of China
Corresponding author: QIU Guan-zhou; Tel: +86-731-8879212; E-mail: qgzfblw@163.com


