J. Cent. South Univ. (2017) 24: 1281-1287
DOI: 10.1007/s11771-017-3533-6
Simultaneous production of hydrogen and volatile fatty acids from anaerobic digestion of Macrocystis pyrifera biomass residues
ZHAO Xiao-xian(赵晓娴)1, 2, FAN Xiao-lei(范晓蕾)1, XUE Zhi-xin(薛志欣)2, YANG Zhi-man(杨智满)1,
YUAN Xian-zheng(袁宪政)1, QIU Yan-ling(邱艳玲)1, GUO Rong-bo(郭荣波)1
1. Shandong Industrial Engineering Laboratory of Biogas Production & Utilization (Key Laboratory of Biofuels),
Qingdao Institute of Bioenergy and Bioprocess Technology, Chinese Academy of Sciences, Qingdao 266101, China;
2. College of Chemical Science and Engineering, Qingdao University, Qingdao 266071, China
 Central South University Press and Springer-Verlag Berlin Heidelberg 2017
Central South University Press and Springer-Verlag Berlin Heidelberg 2017
Abstract:
The Macrocystis pyrifera biomass residues (MPBRs) after extraction of algin could be applied in anaerobic fermentation. The effects of different pretreatment conditions, substrate concentrations and initial pH values on hydrogen and volatile fatty acid (VFA) production during the anaerobic fermentation of MPBRs were evaluated. The optimal pretreatment conditions, substrate concentration, initial pH values were determined as thermo-alkaline pretreatment at 100 °C with 0.1 mol/L NaOH, 40 g/L and 7.0, respectively. Under these conditions, the maximum hydrogen production was 11.38 mL/g (volatile solids, VS), which was approximately 23 times higher than that of untreated MPBRs. Furthermore, the maximum total volatile fatty acid (TVFA) yield was found to be 0.055 g/g (VS) and the VFA mainly consisted of acetic and butyric acids. The results indicate that the yield of TVFA is positively correlated with hydrogen production, and the MPBRs could produce hydrogen and TVFA simultaneously. In addition, thermo-alkaline pretreatment is proven to be the best method for hydrogen and VFA production.
Key words:
Macrocystis pyrifera biomass residues; pretreatment; fermentation; hydrogen;
1 Introduction
M. pyrifera, as one of the largest brown algae, is the main raw material of seaweed industry [1]. Only with the alginic acid as the main product of M. pyrifera, about 70% of the biomass is left without utilization [2]. Therefore, it would have both economic and environmental benefits if M. pyrifera biomass residues (MPBRs) could be converted to bioproducts.
Hydrogen is a clean and renewable energy source, which has been widely recognized as an ideal substitute energy source for fossil fuels [3-5]. Bio-hydrogen production from sustainable resources, such as biomass has been given considerable attention [3, 6-9]. Due to the sufficient sources of raw materials and simple operation, anaerobic fermentation of biomass has been regarded as an important way for producing hydrogen. However, after anaerobic fermentation, only a small part of energy could be recovered as hydrogen, and a large amount of energy is reserved in volatile fatty acids (VFAs) [10-13], such as acetic, propionic and butyric acids. Recently, VFAs have been proposed to be an important platform for fuels and chemicals [14]. Therefore, the simultaneous production of VFAs and hydrogen might be more economically feasible.
To date, hydrogens production by anaerobic fermentation of various marine macro-algaes, such as Ulva lactuca, Porphyra tenera, Undaria pinnatifida, Laminaria japonica, Gelidium amansii [4, 8] and VFA production from L. japonica, Enteromorpha crinita [15] has been intensively studied. However, there is little information regarding simultaneous production of hydrogen and VFA from algal biomass, especially the algin extracted residues. Therefore, in this research, hydrogen and VFA production from MPBRs fermentation is studied, and the effect of different conditions, such as pretreatment methods, substrate concentrations and initial pH values was investigated.
2 Materials and methods
2.1 Inoculum and substrate
Anaerobic digested sludge (ADS) was used as inoculum, which was collected from a 1000 m3 sludge digestion tank in Qingdao Tuandao Waste Water Treatment Plant, China. The pH, total solids (TS) and volatile solids (VS) concentrations of the sludge were 6.9, 14.66 % and 59.44% TS, respectively. Before used as inoculum, the ADS was heated at 95 °C for 30 min to inhibit the bioactivity of methanogens.
MPBRs obtained from Qingdao Gather Great Ocean Seaweed Industrial Co. Ltd (Qingdao, China) were used as substrate. The TS content of the MPBRs was 25.81%, and 62.99% of the TS was VS. The proportions of sugar, protein and lipid in MPBRs were 26.50%, 20.00% and 12.90%, respectively.
2.2 Batch tests
Four series of batch tests were performed at 37 °C in 300 mL serum bottles with a working volume of 100 mL. The thermal-treated sludge with a final concentration of 30 g (TS)/L and medium was added into the serum bottles. The bottles were then purged with high purity N2 for 2 min before sealed with butyl rubber stoppers and placed in a shaker (180 r/min). The medium composition [16] was 5356 mg/L KH2PO4, 164 mg/L K2HPO4·3H2O, 11867 mg/L Na2HPO4·12H2O, 100 mg/L MgCl2·6H2O, 1000 mg/L NaHCO3, 66 mg/L CaCl2·2H2O, 500 mg/L NH4Cl, 1000 mg/L peptone, 600 mg/L yeast extract, 15 mg/L MnSO4·6H2O, 25 mg/L FeSO4·7H2O,5 mg/L CuSO4·5H2O, 0.0125 mg/L CoCl2·5H2O,32 mg/L NiSO4, 23 mg/L ZnCl2, 14 mg/L (NH4)6Mo7O24·4H2O, and 50 mg/L EDTA. All the experiments were carried out triplicate and the results were expressed as means.
Series 1 was designed to study the influence of thermal, thermo-NaOH and acid pretreatment on reducing sugar and soluble chemical oxygen demand (SCOD) production, in which 30 g (TS)/L of the MPBRs was loaded and the initial pH to was set as 7.0. The thermal, thermo-NaOH and acid pretreatments were performed at 100 °C for 2.5 h with 0.05 mol/L NaOH and room temperature for 0.5 h with 0.42 mol/L HCl at the solid to liquid ratio of 3:7, respectively. Series 2 was conducted to investigate the effect of NaOH concentration on hydrogen and VFA production, in which 30 g (TS)/L MPBRs were pretreated with 0, 0.05, 0.1, 0.2 and 0.4 mol/L NaOH at 100 °C for 2.5 h respectively and the initial pH value was 7.0. Series 3 was used to investigate the effect of the substrate concentration on hydrogen and VFA production. The substrates used in this series were 30, 40, 60, 100 g/L, respectively, all of which were pretreated with 0.1 mol/L NaOH at 100 °C for 2.5 h and the initial pH value was 7.0. Series 4 was used to examine the effect of initial pH (from 4.0 to 8.0 with an increment of 1.0) on hydrogen and VFA production, in which MPBRs were pretreated with 0.1 mol/L NaOH at 100 °C for 2.5 h and the substrate concentration was 40 g/L.
2.3 Analytical methods
The hydrogen production is calculated as follows:
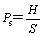
where H is the cumulative hydrogen production (mL), Ps is hydrogen production (mL/g, VS) and S is the initial amount (g, VS). The volume of biogas produced was measured using a water displacement method. The fraction of H2 was periodically analyzed by a gas chromatograph (GC, type SP6890, Shandong, China) equipped with a thermal conductivity detector (TCD). High-purity nitrogen (99.999%) was used as the carrier gas with a flow rate of 20 mL/min. The temperatures of the injection port, oven and detector were 100, 50 and 100 °C, respectively [17]. VFA was determined by a gas chromatograph (450GC, VARIAN, USA) equipped with a flame ionization detector (FID) and a 30 m×0.25 mm× 0.25 μm FFAP fused silica capillary column. High-purity nitrogen was used as the carrier gas with a flow rate of 1 mL/min. The temperatures of the injector and detector were 220 and 240 °C, respectively. The programmed temperatures in the column oven were as follows: 80 °C held for 1 min; 25 °C/min to 120 °C; then 30 °C/min to 135 °C; 15 °C/min to 180 °C, held for 1 min [18]. The pH of the solution was measured by a pH meter (Sartorius, Germany). The TS and VS were determined according to standard methods [19].
3 Results and discussion
3.1 Reducing sugar and SCOD production from MPBRs under different pretreatment conditions
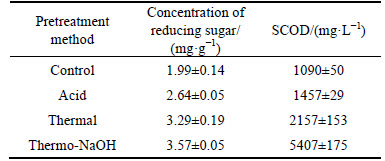
MPBRs after treatment with thermal, thermo-NaOH and HCl were applied to anaerobic fermentation. The reducing sugar and SCOD production are shown in Table 1. Compared with that of raw MPBRs without pretreatment, the reducing sugar and SCOD productions of all pretreated residues were significantly improved. In addition, the biogas produced was free of methane in all tests, indicating that the methanogens have been severely suppressed. When the MPBRs were treated with thermo- NaOH, the concentration of reducing sugar and SCOD productions reached to the most as 3.57 mg/g (VS) and 5407 mg/L, respectively. Similar results were also reported in the lipid-extracted microalgal (Scenedesmus obliquus) biomass residues, in which, a thirteen-fold increase in hydrogen yield was obtained after thermo- NaOH pretreatment [18]. This might be due to that the main components of MPBRs are some water-insoluble cell wall fibers and proteins. Since the hydrogen-bonds were very sensitive to temperature, most of the hydrogen-bonds of the polymers would be destroyed under high temperature [4], and then alkali could catalyze the hydrolysis of glucosidic bonds more easily [20]. As a result, more reducing sugar was released in the thermo- NaOH treated MPBRs than the others (Table 1). In addition, when the polysaccharide backbone was broken, the blocked protein might be released [21], which consequently resulted in the higher SCOD (Table 1). Moreover, it was recently found that high pH (pH=12) value could cause the unfolding of protein, damage the protein hydrogen bonding networks, and destroy the disulfide-bridges, which would increase the susceptibility of protein to protease in anaerobic fermentation of protein wastewater [22]. Therefore, we supposed thermo-NaOH treatment as the optimal pretreatment method for anaerobic fermentation of MPBRs among the three pretreatment methods in this work.
Table 1 Concentration of reducing sugar and SCOD production from MPBRs under different pretreatment conditions
3.2 Hydrogen and VFA production from MPBRs pretreated with different NaOH concentrations
Excess NaOH in pretreatment will greatly increase the cost of pretreatment. Therefore, hydrogen and VFA production of pretreated MPBRs with different NaOH concentrations were studied. As shown in Table 2, the cumulative hydrogen production of MPBRs was greatly influenced by NaOH concentration, which increased from 0.96 to 3.45 mg/g (VS) according to the NaOH concentration from 0 to 0.1 mol/L, and declined when the NaOH concentration increased further. Although the reducing sugar and SCOD did not decrease when the NaOH concentration was higher than 0.1 mol/L, obvious reduction of hydrogen yield was observed. However, YANG et al [16] reported 0.2 mol/L as the optimal NaOH concentration in lipid-extracted microalgal (S. obliquus) biomass residues. Different Na contents of the feedstock might be responsible for the difference. While the Na+ content was determined to be 6553 mg/kg (TS) in MPBRs in this work, which might be much higher than that in lipid-extracted microalgal (S. obliquus) biomass residues. Similar trends were also found in the production of total volatile fatly acid (TVFA) and VS conversion efficiency (Table 2, Fig. 1(a)). The maximum TVFA convert ratio (0.051 g/g, VS) was also obtained at 0.1 mol/L NaOH, which showed 75.86% improvement compared to the control (0 mol/L NaOH). It is indicated that although the high NaOH content is helpful for the release of reducing sugar and SCOD, only Na+ with suitable concentration (about 5994 mg/L) would be helpful for both the production of hydrogen and VFA. In addition, VFA analysis results show that the acetic and butyric acids are the main metabolites in all the fermentation broth, suggesting that an acetic acid type fermentation occurred.
3.3 Hydrogen and VFA production at different substrate concentrations
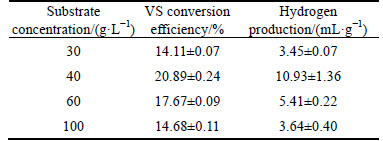
Substrate concentration plays an important role in the stability and progress of anaerobic digestion [9, 23, 24], because it might affect the bacterial structure, biological activity and metabolic characteristics of bacteria in the hydrogen production system, which determines the hydrogen production capacity of fermentation bacteria [17, 25-27]. In this work, hydrogen and VFA production of MPBRs at different substrate concentrations (30-100 g/L) were studied. As shown in Table 3 and Fig. 1(b), the maximum of both hydrogen and TVFA yields was obtained at substrate concentration of 40 g/L, which were 10.93 mL/g (VS) and 0.053 g/g (VS), respectively. When the substrate concentration was higher than 40 g/L, further increase in the substrate concentration resulted in the decrease in the yield of hydrogen and TVFA, which suggested that the substrate was excessive for the inoculum, and both substrate and product inhibition might become obvious [28]. While when the substrate concentration was less than 40 g/L, the lower hydrogen and TVFA yield might be due to the substrate loss caused by microbe assimilation and the substrate limitation for effective metabolism and further hydrogen production process [29]. Similar results were also reported by ANTONOPOULOU et al [30] that the increase in the substrate concentration firstly increased the hydrogen production and then decreased the amount of hydrogen produced and the rate of production when substrate concentrations exceeded certain threshold levels [30].
Table 2 Yields of reducing sugar, SCOD and hydrogen together with VS conversion efficiency in set-ups pretreated with various NaOH concentrations (Dataa is determined after thermo-NaOH pretreatment, VS; Datab is determined after anaerobic fermentation, VS)
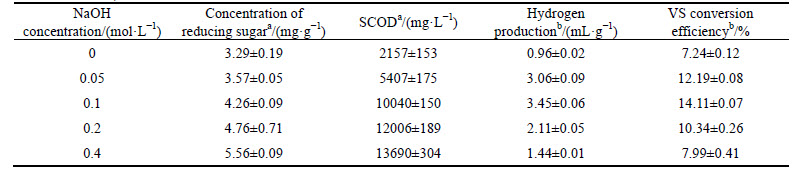
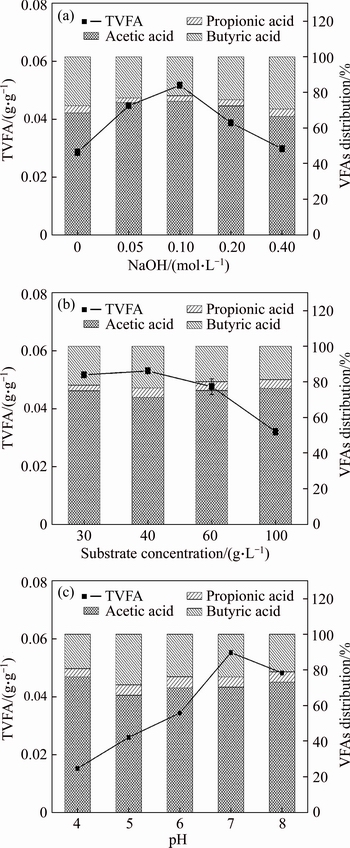
Fig. 1 Effect of NaOH concentration (a), substrate concentration (b) and initial pH values (c) on distribution of VFAs (VS)
At the same time, VS conversion efficiency reached to the highest (20.89%) when the substrate concentration was 40 g/L (Table 3). The distribution of VFA is shown in Fig. 1(b) and the contents of acetic acid was 71.44% to 76.45%, indicating that acetic acid still is the dominated species in VFA and substrate concentration changes do not affect the fermentation type though it might have obvious effect on the activity of anaerobic microbial obviously (Fig. 1(b)).
Table 3 VS conversion efficiency and yield of hydrogen (VS) after anaerobic fermentation with different substrate concentrations
3.4 Hydrogen and VFA production with different initial pH values
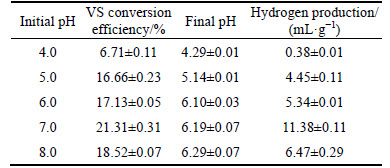
In anaerobic fermentation system, the changes of pH values affect not only the enzyme activity involved in the metabolic process, but also the structure and abundance of dominant populations in the reactor, as well as the fermentation type of hydrogen production in reactor [31]. However, due to the complexity of the substrate and hydrogen-producing bacterial diversity, the optimal pH values of different reaction systems were not consistent. For example, the optimal initial pH value of alkali treated sludge without any inoculum was 11.0, whereas, that of thermal treated starch with bacteria (enriched Clostridium pasteurianum) as inoculum was between 7.0 and 8.0, which was used for hydrogen production in this work [32]. It was reported that lipid-extracted microalgal (S. obliquus) biomass residues have the highest hydrogen production rate when the initial pH value was 6.5 [33]. In this work, it was found that the yield of hydrogen and TVFA increased corresponding to the initial pH values increasing from 4.0 to 7.0, and then decreased when the initial pH value increased further (Table 4, Fig. 1(c)). The maximum yields of hydrogen and TVFA were 11.38 mL/g (VS) and 0.055 g/g (VS), respectively. At the same time, VS conversion efficiency reached 21.31% (Table 4). When the initial pH value was less than 5.0, the fermentation system produced only a small amount of hydrogen. The lowest hydrogen yield (0.38 mL/g VS) and VS conversion efficiency (6.71%) were obtained at the initial pH value of 4.0. This might be caused by the deviation of initial pH value from the optimal pH value, which leads to the poor growth of the hydrogen producing bacteria and the decrease of hydrogen production [34]. The final pH values were 4.29-6.30 corresponding to the initial pH values of 4.0-8.0 (Table 4). Correlation between the initial and final pH values has also been reported by other researchers [32, 33]. In addition, levels of TVFA concentration also increased with the initial pH values from 4.0 to 7.0 and then decreased with the initial pH values from 7.0 to 8.0, and the maximum TVFA concentration was 1.39 g/L when the initial pH value was 7.0 (Fig. 2). Furthermore, the yield of acetic and butyric acids also increased with the initial pH values from 4.0 to 7.0 and then decreased with the initial pH values from 7.0 to 8.0. At the end of the fermentation process, the contents of acetic acid accounted for more than 65% of the TVFA in all the set-ups, which suggested that acetic acid was the main intermediate metabolite (Fig. 1(c)). It should be pointed that significant level of propionic acid (53-80 mg/L) was detected at the initial pH values range of 6.0-8.0, indicating that propionic acid-producing bacteria might involve in MPBRs fermentation, which was consistent with the previous report that propionic acid bacteria mainly appeared when pH value was above 5.0 [35]. The propionic acid fermentation is known to produce propionic acid at the expense of hydrogen [36]. Therefore, the inhibition of propionic acid production should be considered in the future studies for more hydrogen.
Table 4 VS conversion efficiency, final pH values and yield of hydrogen (VS) after anaerobic fermentation with various initial pH values
It is interesting that the yield of TVFA and hydrogen shows significantly positive correlations under all the above conditions (Tables 2, 3, 4 and Fig. 3). A similar phenomenon was also found in Microcystis spp fermentation [37]. The highest hydrogen yield is obtained about 72 h later and then it decreases with time, while the yield of TVFA tends to be stable after 72 h. It is indicated that both the highest yields of hydrogen and TVFA of the MPBRs could be obtained simultaneously. The decrease of hydrogen yield after 72 h indicates that hydrogen-consuming bacteria, such as homoacetogens,might exist in the fermentation system since methanogens have been severely suppressed. It was once reported that homoacetogens could reduce hydrogen conversion rate of glucose and the specific hydrogen production rate by the activated sludge in terms of mixed liquor volatile suspended solids (MLVSS) by 31% and 34%, respectively, with no obvious negative influence on the yield of TVFA [38]. So, it is speculated that inhibition of homoacetogens might also result in higher H2 production in MPBRs.
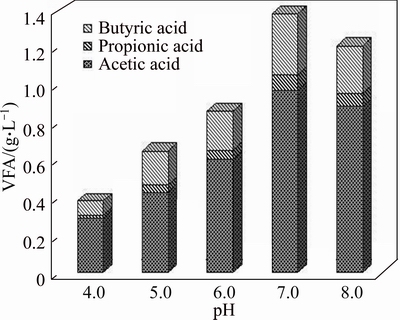
Fig. 2 VFA composition with various initial pH values
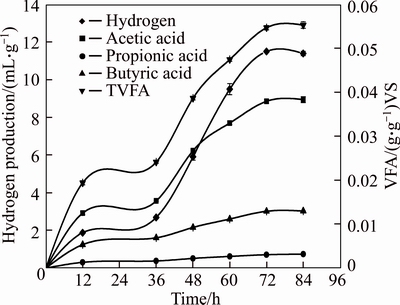
Fig. 3 Variation of yield of hydrogen and VFA with digestion time
It was reported that fermentation hydrogen production from Laminaria japonica was investigated under mesophilic condition without any pretreatment, and 71.4 mL/g (VS) of hydrogen yield was achieved at a substrate concentration of 20 g/L chemical oxygen demand (COD), with an initial pH of 7.5 [39]. When red algae was hydrolyzed at 150 °C for 15 min and detoxified by activated carbon, the hydrogen production was 53.5 mL/g (VS) [8]. But the maximum hydrogen production was only 7.13 mL/g (VS) at the inoculum– substrate ratio (ISR) value of 0.3 with Chlorella sp. as substrate [9]. The VFA yield of Enteromorpha crinita that was pretreated with Vibrio harveyi reached to 0.024 g/g (VS) [15]. However, it should be noted that the yield of hydrogen and TVFA and VS conversion efficiency of MPBRs were only 11.38 mL/g (VS),0.055 g/g (VS) and 21.31%, respectively, indicating that a lot of biomass has not been used efficiently. The partial pressure of H2 has been regarded as an extremely important factor for continuous H2 synthesis. Hydrogen synthesis pathways are sensitive to H2 concentrations and are subjected to end-product inhibition [40]. As H2 concentrations increase, H2 synthesis decreases and metabolic pathways shift to production of more reduced substrates such as lactate, ethanol, acetone, butanol, or alanine. Taking into account that there is no real-time separation of hydrogen in our batch experiment, it is speculated that hydrogen accumulated in the headspace might cause feedback effects on the fermentation. Similar results were also found by ZENG et al [37]. So it was speculated that hydrogen and VFA production could be further promoted by real-time separation of hydrogen. In addition, considerable amounts of heavy metals were also determined in MPBRs, such as Mg (4828 mg/kg, TS), and Ca (86745 mg/kg, TS). Studies have found that hydrogen production could be promoted when the concentration of Ca2+ reached to 75-150 mg/L, while Ca2+ concentration more than 300 mg/L would inhibit the production of hydrogen [41]. Obviously, in this work, under the optimized conditions, Ca2+ concentration reaches to about 3487.76 mg/L, which is far more than 300 mg/L. So, Ca2+ is thought to be one of the major inhibition factors. In addition, in the fermentation process of corn stalks, the hydrogen yield and hydrogen production rate are also found to decline when Mg2+ concentration reaches to more than 10 mg/L [42]. However, under the optimized conditions, Mg2+ concentration reaches to about 204.94 mg/L, which is also far more than 10 mg/L in this work, indicating that it might be another important inhibiting factor. Therefore, the heavy metals are speculated to be the major obstacle for efficient degradation of MPBRs and proper metal chelators might be helpful to improve the efficiency of the MPBRs system.
4 Conclusions
This work demonstrates the availability of MPBRs as the feedstock for hydrogen and VFA production. The results show that MPBRs could be effectively fermented after thermal-NaOH pretreatment, and the yield of TVFA is positively correlated with hydrogen production, indicating that MPBRs could be used to produce hydrogen and TVFA simultaneously by anaerobic fermentation. The optimal pretreatment conditions, substrate concentration, initial pH value are determined to be thermal-NaOH pretreatment at 100 °C with 4 g/L NaOH, 40 g/L and 7.0, respectively. Under these conditions, the maximum yields of hydrogen and TVFA are 11.38 mL/g (VS) and 0.055 g/g (VS), respectively, and the highest VS conversion efficiency reached to 21.31%. Nevertheless, the VS conversion efficiency of MPBRs is still lower compared with other algal biomass, which is expected to be greatly improved by adding metal chelators and real-time separation of hydrogen.
References
[1] HORN S J. Bioenergy from brown seaweeds[M]. Norway: Department of Biotechnology Norwegian University of Science and Technology NTNU Trondheim, 2000.
[2] HORN S J, MOEN E, OSTGAARD K. Direct determination of alginate content in brown algae by near infrared (NIR) spectroscopy [J]. J Appl Phycol, 1999, 11(1): 9-13.
[3] LIU Hong-yan, WANG Guang-ce. Fermentative hydrogen production from macroalgae Laminaria japonica using anaerobic mixed bacteria [J]. Int J Hydrogen Energ, 2014, 39(17): 1-6.
[4] PARK J I, LEE J, SIM S J, LEE J H. Production of hydrogen from marine macroalgae biomass using anaerobic sewage sludge microflora [J]. Biotechnol Bioproc E, 2009, 14: 307-315 .
[5] WANG Yu, WANG Hui, FENG Xiao-qiong, WANG Xiao-fang, HUANG Jian-xin. Biohydrogen production from cornstalk wastes by anaerobic fermentation with activated sludge [J]. Int J Hydrogen Energ, 2010, 35(7): 3092-3099.
[6] GURUNG A, van GINKEL S W, KANG W C, QAMBRANI N A, OH S E. Evaluation of marine biomass as a source of methane in batch tests: A lab-scale study [J]. Energy, 2010, 43(1): 396-401.
[7] MARQUEZ G P, REICHARDT W T, AZANZA R V, KLOCKE M, MONTANO M N. Thalassic biogas production from sea wrack biomass using different microbial seeds: Cow manure, marine sediment and sea wrack-associated microflora [J]. Bioresource Technol, 2013, 133: 612-617.
[8] PARK J H, YOON J J, PARK H D, KIM Y J, LIM D J, KIM S H. Feasibility of biohydrogen production from Gelidium amansii [J]. Int J Hydrogen Energ, 2011, 36: 13997-14003 .
[9] SUN Jing-xian, YUAN Xian-zheng, SHI Xiao-shuang, CHU Chun-feng, GUO Rong-bo, KONG Hai-nan. Fermentation of Chlorella sp. for anaerobic bio-hydrogen production: Influences of inoculum-substrate ratio, volatile fatty acids and NADH [J]. Bioresource Technol, 2011, 102: 10480-10485.
[10] HAWKES F R, FORSEY H, PREMIER G C, DINSDALE R M, HAWKES D L, GUWY A J, MADDY J, CHERRYMAN S, SHINE J, AUTY D. Fermentative production of hydrogen from a wheat flour industry co-product [J]. Bioresource Technol, 2008, 99: 5020-5029.
[11] KAPDAN I K, KARGI F. Bio-hydrogen production from waste materials [J]. Enzyme Microb Tech, 2006, 38(5): 569-582 .
[12] HAN S K, SHIN H S. Biohydrogen production by anaerobic fermentation of food waste [J]. Int J Hydrogen Energ, 2004, 29(6): 569-577.
[13] LAY J J, FAN K S, CHANG J, KU C H. Influence of chemical nature of organic wastes on their conversion to hydrogen by heat-shock digested sludge [J]. Int J Hydrogen Energ, 2003, 28(12): 1361-1367 .
[14] HOLTZAPPLE M T, GRANDA C B. Carboxylate platform: The MixAlco process part 1: Comparison of three biomass conversion platforms [J]. Appl Biochem Biotech, 2009, 156(1-3): 95-106 .
[15] PHAM T N, UM Y, YOON H H. Pretreatment of macroalgae for volatile fatty acid production [J]. Bioresource Technol, 2013, 146: 754-757 .
[16] YANG Zhi-man, GUO Rong-bo, XU Xiao-hui, FAN Xiao-lei, LI Xiao-ping. Thermo-alkaline pretreatment of lipid-extracted microalgal biomass residues enhances hydrogen production [J]. J Chem Technol Biot, 2010, 86(3): 454-460.
[17] YANG Zhi-man, GUO Rong-bo, XU Xiao-hui, FAN Xiao-lei, LUO Sheng-jun. Fermentative hydrogen production from lipid-extracted microalgal biomass residues [J]. Appl Energ, 2011, 88: 3468-3472 .
[18] YANG Zhi-man, GUO Rong-bo, XU Xiao-hui, FAN Xiao-lei, LUO Sheng-jun. Enhanced hydrogen production from lipid-extracted microalgal biomass residues through pretreatment [J]. Int J Hydrogen Energ, 2010, 35: 9618-9623.
[19] APHA. Standard methods for the examination of waste and wastewater [M]. 20th ed. Washington, DC: American Public Health Association, 2000.
[20] BACCAY R A, HASHIMOTO A G. Acidogenic and methanogenic fermentation of causticized straw [J]. Biotechnol Bioeng, 1984, 26(8): 885-891.
[21] PENAUD V, DELGENES J P, MOLETTA R. Thermo-chemical pretreatment of a microbial biomass: Influence of sodium hydroxide addition on solubilization and anaerobic biodegradability [J]. Enzyme Microb Tech, 1999, 25(3-5): 258-263.
[22] XIAO Nai-dong, CHEN Yin-guang, CHEN Ai-hui, FENG Lei-yu. Enhanced bio-hydrogen production from protein wastewater by altering protein structure and amino acids acidification type [J]. Sci Rep-UK, 2014, 4: 3992.
[23] LI Yan, HUA Dong-liang, ZHANG Jie, GAO Min-tian, ZHAO Yu-xiao, XU Hai-peng, LIANG Xiao-hui, JIN Fu-qiang, ZHANG Xiao-dong. Influence of inoculum to substrate ratios (ISRs) on the performance of anaerobic digestion of algal residues [J]. Ann Microbiol, 2013, 64(3): 955-960.
[24] DECHRUGSA S, KANTACHOTE D, CHAIPRAPAT S. Effects of inoculum to substrate ratio, substrate mix ratio and inoculum source on batch co-digestion of grass and pig manure [J]. Bioresource Technol, 2013, 146: 101-108.
[25] GINKEL S V, SUNG S, LAY J J. Biohydrogen production as a function of pH and substrate concentration [J]. Environ Sci Technol, 2001, 35: 4726-4730.
[26] van NIEL E W, CLAASSEN P A, STAMS A J. Substrate and product inhibition of hydrogen production by the extreme thermophile, caldicellulosiruptor saccharolyticus [J]. Biotechnol Bioeng, 2003, 81(3): 255-262.
[27] KIM S H, HAN S K, SHIN H S. Effect of substrate concentration on hydrogen production and 16S rDNA-based analysis of the microbial community in a continuous fermenter [J]. Process Biochem, 2006, 41: 199-207.
[28] PAN C M, FAN Y T, HOU H W. Fermentative production of hydrogen from wheat bran by mixed anaerobic cultures [J]. Ind Eng Chem Res, 2008, 47: 5812-5818.
[29] PRAKASHAM R S, BRAHMAIAH P, SATHISH T, SAMBASIVA RAO K R S. Fermentative biohydrogen production by mixed anaerobic consortia:Impact of glucose to xylose ratio [J]. Int J Hydrogen Energ, 2009, 34: 9354-9361.
[30] ANTONOPOULOU G, GAVALA H N, SKIADAS I V, LYBERATOS G. Effect of substrate concentration on fermentative hydrogen production from sweet sorghum extract [J]. Int J Hydrogen Energ, 2011, 36: 4843-4851.
[31] GONG Man-li, REN Nan-qi, TANG Ji. Start-up and continuous operation of bio-hydrogen production reactor at pH 5 [J]. Environ Sci, 2005, 26(2): 177-180.
[32] LIU Guang-zhen, SHEN Jian-quan. Effects of culture and medium conditions on hydrogen production from starch using anaerobic bacteria [J]. J Biosci Bioeng, 2004, 98: 251-256.
[33] KHANAL S. Biological hydrogen production: Effects of pH and intermediate products[J]. Int J Hydrogen Energ, 2004.
[34] CHEONG D, HANSEN C. Acidogenesis characteristics of natural, mixed anaerobes converting carbohydrate-rich synthetic wastewater to hydrogen [J]. Process Biochem, 2006, 41: 1736-1745.
[35] HUSSY I, HAWKES F R, DINSDALE R, HAWKES D L. Continuous fermentative hydrogen production from a wheat starch co-product by mixed microflora [J]. Biotechnol Bioeng, 2003, 84(6): 619-626.
[36] COHEN A, DISTEL B, van DEURSEN A. Role of anaerobic spore-forming bacteria in the acidogenesis of glucose: Changes induced by discontinuous or low-rate feed supply [J]. Anton Leeuw Int J G, 1985, 51(2): 179-192.
[37] ZENG Shu-juan, YUAN Xian-zheng, SHI Xiao-shuang, QIU Yan-ling. Effect of inoculum/substrate ratio on methane yield and orthophosphate release from anaerobic digestion of Microcystis spp [J]. J Hazard Mater, 2010, 178(1-3): 89-93.
[38] LI Jian-zheng, XU Ying-ping, ZHANG Li-guo. Homoacetogens and hydrogen consumption in anaerobic activated sludge bio-hydrogen production system [J]. Sci Technol Rev, 2011, 24: 29-32.(in Chinese)
[39] SHI Xue-qing, JUNG K W, KIM D H, AHN Y T, SHIN H S. Direct fermentation of Laminaria japonica for biohydrogen production by anaerobic mixed cultures [J]. Int J Hydrogen Energ, 2011, 36: 5857-5864.
[40] LEVIN D. Biohydrogen production: Prospects and limitations to practical application [J]. Int J Hydrogen Energ, 2004, 29(2): 173-185.
[41] CHANG F Y, LIN C Y. Calcium effect on fermentative hydrogen production in an anaerobic up-flow sludge blanket system [J]. Water Sci Technol, 2006, 54(9): 105-112.
[42] SUN Xue-xi, LI Tao, JI Xin-jing. Parameter effects and mechanism of fermentative bio-hydrogen production from cornstalks [J]. Mod Chem Ind, 2010, 30(6): 60-62. (in Chinese)
(Edited by FANG Jing-hua)
Cite this article as:
ZHAO Xiao-xian, FAN Xiao-lei, XUE Zhi-xin, YANG Zhi-man, YUAN Xian-zheng, QIU Yan-ling, GUO Rong-bo. Simultaneous production of hydrogen and volatile fatty acids from anaerobic digestion of Macrocystis pyrifera biomass residues [J]. Journal of Central South University, 2017, 24(6): 1281-1287.
DOI:https://dx.doi.org/10.1007/s11771-017-3533-6Foundation item: Project(2015GSF117016) supported by the Key Research &Development Project of Shandong Province, China; Project(2014BAC31B01) supported by the National Science and Technology Pillar Program during the 12th Five-Year Plan Period; China Project(2015GSF115037) supported by the Foundation of Key Program of Science and Technology of Shandong Province, China
Received date: 2015-11-05; Accepted date: 2016-03-03
Corresponding author: GUO Rong-bo, Professor; Tel: +86-13791936409; E-mail: guorb@qibebt.ac.cn
Abstract: The Macrocystis pyrifera biomass residues (MPBRs) after extraction of algin could be applied in anaerobic fermentation. The effects of different pretreatment conditions, substrate concentrations and initial pH values on hydrogen and volatile fatty acid (VFA) production during the anaerobic fermentation of MPBRs were evaluated. The optimal pretreatment conditions, substrate concentration, initial pH values were determined as thermo-alkaline pretreatment at 100 °C with 0.1 mol/L NaOH, 40 g/L and 7.0, respectively. Under these conditions, the maximum hydrogen production was 11.38 mL/g (volatile solids, VS), which was approximately 23 times higher than that of untreated MPBRs. Furthermore, the maximum total volatile fatty acid (TVFA) yield was found to be 0.055 g/g (VS) and the VFA mainly consisted of acetic and butyric acids. The results indicate that the yield of TVFA is positively correlated with hydrogen production, and the MPBRs could produce hydrogen and TVFA simultaneously. In addition, thermo-alkaline pretreatment is proven to be the best method for hydrogen and VFA production.


