J. Cent. South Univ. (2017) 24: 2266-2274
DOI: https://doi.org/10.1007/s11771-017-3637-z

Extracting reaction mechanism analysis of Zn and Si from zinc oxide ore by NaOH roasting method
CHEN Bing(陈兵)1, 2, 3, SHEN Xiao-yi(申晓毅)1, 3, GU Hui-min(顾惠敏)1, 3,
SHAO Hong-mei(邵鸿媚)1, ZHAI Yu-chun(翟玉春)1, MA Pei-hua(马培华)1
1. School of Metallurgy, Northeastern University, Shenyang 110819, China;
2. Science and Technology Laboratory, Academy of Environmental Sciences, Panjin 124010, China;
3. Liaoning Key Laboratory for Metallurgical Sensor and Technology, Northeastern University,Shenyang 110819, China
 Central South University Press and Springer-Verlag GmbH Germany 2017
Central South University Press and Springer-Verlag GmbH Germany 2017
Abstract:
The orthogonal test was used to optimize the reaction conditions of roasting zinc oxide ore with NaOH aiming to comprehensively utilize zinc oxide ore. The optimized reaction conditions were molar ratio of NaOH to zinc oxide ore 6:1, roasting temperature 450 °C, holding time 150 min. The molar ratio of NaOH to zinc oxide ore was the most predominant factor affecting the extraction ratios of zinc oxide and silica. The mineral phase transformations were investigated by testing the phases of specimens obtained at different temperatures. The process was that silica reacted with molten NaOH to form Na2SiO3 at first, then transformed into Na4SiO4 with temperature rising. ZnCO3 and its decomposing product ZnO reacted with NaOH to form Na2ZnO2. Na2ZnSiO4 was also obtained. The reaction rate was investigated using unreacted shrinking core model. Two models used were chemical reaction at the particle surface and diffusion through the product layer. The results indicated that the reaction rate was combine-controlled by two models. The activation energy and frequency factor were obtained as 24.12 kJ/mol and 0.0682, respectively.
Key words:
zinc oxide ore; NaOH roasting method; reaction process; reaction mechanism; kinetics;
1 Introduction
As an important nonferrous metal, zinc is widely used in galvanizing and battery field [1, 2]. Zinc is mainly produced from sulfide ore so far. However, due to the excess exploration and the rising demand for zinc, the zinc sulfide ore is gradually exhausted [3–6]. So, many researchers have to focus on the utilization of zinc oxide ore [7–9]. Zinc oxide ore is the secondary ore of zinc, which presents in a variety of oxide minerals, including Zn2SiO4, Zn4(Si2O7)(OH)2·H2O, ZnCO3, 2ZnCO3·2Zn(OH)2 and so on [10–13]. Typical metallurgical routes for extracting zinc from zinc oxide ore include pyrometallurgical methods, hydrometallurgical methods and their combination [2]. The routes of pyrometallurgical methods are to product spelter or crude zinc oxide by reducing zinc oxide in ore at 1000–1200 °C. However, due to high energy consumption and heavy pollution, pyrometallurgical methods are gradually losing their attraction [14, 15]. Hydrometallurgical methods include acid leaching, alkaline leaching and ammonia leaching. Every one has its advantages, but the challenges still exist. In acid leaching, silica gel is easily generated, which makes the following filtration difficult [16, 17]. When alkaline leaching is carried out, SiO2 and PbO with ZnO will dissolve in the alkaline solution together, so additional work is needed to separate SiO2, ZnO and PbO [18, 19]. When ammonia leaching is adopted, the vessels must be hermetically sealed because the volatile ammonia is a great threat to operator’s health and environment [20, 21].
Many researchers have focused on the improvement of current zinc extracting methods from complex zinc oxide ore. NaOH roasting technology is an effective method and can be carried out simply in air at low roasting temperature with pervasive equipments. In this work, NaOH roasting was adopted to extract ZnO, SiO2 and PbO from zinc oxide ore to produce Na2ZnO2, Na4SiO4 and Na6PbO5. The extraction ratios of ZnO and SiO2 were used as evaluation index in the orthogonal test.The mineral phase transformation was analyzed, and the reaction mechanism of roasting process was discussed.
2 Experimental
2.1 Materials and characterization
The zinc oxide ore used in experiments was obtained from a mine in Yunnan Province, China. The roast-alkali was industrial NaOH and the solvent was distilled water.
2.2 Procedure
First, the dried lump zinc oxide ore was crushed and ground to 74 mm (200 mesh) powders. Then the powders were mixed with industrial NaOH according to a certain molar ratio. Some mixture was weighed, put into a crucible, then placed in muffle furnace, heated to the setting temperature and held for a period of time. After calcination, the sample was taken out and leached in 80 °C water for 60 min and filtered, referring to Ref. [22]. The contents of Zn and Si both in solution and filter residue were examined by titration method, which were used to calculate the extraction ratios of zinc oxide and silica.
In kinetic experiments, the muffle furnace was heated to the setting temperature first, and then a series of crucibles with reactant mixture were put simultaneously and quickly into the furnace. After the temperature was stable, the crucibles were taken out one by one at a predetermined time interval and cooled rapidly in order to reduce the measurement error. The contents of Zn and Si were examined and the extraction ratios were calculated.
It is noteworthy that the molar ratio of NaOH to zinc oxide ore was the molar ratio of NaOH to molar sum of zinc oxide, silica and lead oxide in zinc oxide ore.
3 Results and discussions
3.1 Characterization of zinc oxide ore
XRD pattern and SEM image of zinc oxide ore are shown in Fig. 1. It is found that the main mineral phases in zinc oxide ore are ZnCO3, SiO2, Fe3O4, CaCO3, PbCO3 and CaSO4·2H2O (see Fig. 1(a)). The particles of the powder were irregular and distributed in a wide size range (see Fig. 1(b)).
The main chemical compositions in zinc oxide ore are listed in Table 1. The content of ZnO is 25.36%, which is the highest in zinc ore. The contents of SiO2, PbO, Fe2O3 and CO2 are 18.84%, 5.13%, 20.07% and 15.29%, respectively. The content of CaO is 5.96%, which is undesired. Because it will transform into insoluble Ca2SiO4, the extraction ratio of silica should be reduced. CO2 comes from ZnCO3, PbCO3 and CaCO3, which is confirmed by XRD pattern.

Fig. 1 XRD pattern (a) and SEM image (b) of zinc oxide ore
Table 1 Chemical contents of main compositions in zinc oxide ore (mass fraction, %)

3.2 Orthogonal test
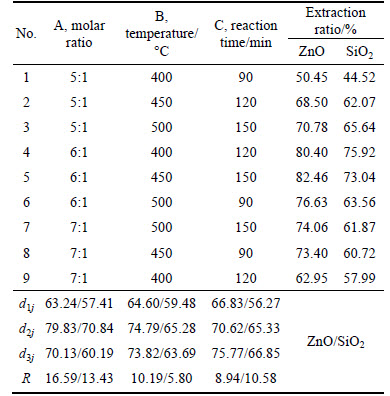
Based on previous experimental results [6], the orthogonal test was adopted to optimize the reaction conditions of molten NaOH roasting zinc oxide ore. The L9(34) tabulation consisting of four factors and three levels was chosen, as listed in Table 2. Three factors were molar ratio of NaOH to zinc oxide ore, roasting temperature, holding time. The results are listed in Table 3. The effects of the molar ratio of NaOH to zinc oxide ore, roasting temperature, holding time on the extraction ratios of ZnO and SiO2 were notable. Among the three factors, the molar ratio had the most significant influence on the extraction. Roasting temperature had a less effect on ZnO extraction. But it was the weakest on SiO2 extraction. The optimized reaction conditions for extracting zinc oxide and silica were as follows: molar ratio of NaOH to zinc oxide ore 6:1, roasting temperature 450 °C, holding time 150 min.
From 400 °C to 500 °C, the extraction ratio of ZnO kept increasing because the reaction between zinc oxide ore and NaOH was enhanced with the temperature rising. The extraction ratio of zinc oxide increased with rising molar ratio of NaOH to zinc oxide ore. This was because when the NaOH was insufficient, the reaction between zinc oxide ore and NaOH was inadequate. Conversely, when NaOH was enough, the reaction between zinc oxide ore and NaOH was improved due to the increasing contact area. Prolonging holding time increased the extraction ratio of zinc oxide. However, if the holding time is too long, it will cause energywaste as well as production efficiency decreasing. Thus, the holding time of 150 min was selected.
Table 2 Factors and levels of orthogonal test

Table 3 Results of orthogonal test
3.3 Reaction process analysis
To illuminate the chemical reaction mechanism and mineral phase transformations in the roasting process, the phases of the specimens obtained at 300, 400, 500 and 600 °C were examined by XRD analysis. Figures 2(a)–(d) shows the XRD patterns of these specimens. It obviously showed that the quartz SiO2 did not react with NaOH. The amorphous silica reacted with molten NaOH to form Na2SiO3 at first, followed by the transformation from Na2SiO3 to Na4SiO4 with rising roasting temperature. ZnCO3 and its decomposition product ZnO reacted with NaOH to form Na2ZnO2. Na2ZnSiO4 was synthesized and stably existed when the roasting temperature was above 400 °C. Iron oxides of Fe2O3 and Fe3O4 were relatively stable. NaOH·H2O was detected due to the significant water-absorbing capability of NaOH. NaHCO3 was attributed to CO2 absorption by wet NaOH. The existences of Na2ZnSiO4 and Ca2SiO4 accounted for why the extraction ratios of ZnO and SiO2 were not high.

Fig. 2 XRD patterns of specimens obtained at 300 °C (a), 400 °C (b), 500 °C (c) and 600 °C (d)
3.4 Kinetic study
The melting point of NaOH is 318 °C and the extracting reaction of Zn and Si occur above 400 °C, so the extracting reactions between NaOH and zinc oxide ore should be liquid–solid reaction, which can be investigated by unreacted shrinking core model [2]. If the zinc oxide ore powders are looked as spherical particles, the reaction process may be controlled by two different models. When the reaction rate is controlled by chemical reaction occurring at particle surface [23], Eq. (1) is established. When the reaction rate is controlled by diffusion in solid product layer, Eq. (2) is established.
 (1)
(1)
 (2)
(2)
where t is reaction time (min); α is extracting fraction of zinc oxide or silica; kc and kp are reaction rate constants. 3.4.1 Effect of NaOH to zinc oxide ore molar ratio
The influence of NaOH to zinc oxide ore molar ratio on extraction ratios of zinc oxide and silica was investigated. The roasting temperature was kept constant at 450 °C. The results are plotted in Fig. 3.
The curves indicated that as molar ratio of NaOH to zinc oxide ore increased, the extraction ratios of zinc oxide and silica increased. Increasing dosage of NaOH raised the extraction ratios, which was conducive to augment the surface contact area. Within 120 min, the extraction ratios of zinc oxide and silica were kept increasing. The maximum values of zinc oxide and silica extraction ratios at the molar ratio of NaOH to zinc oxide ore 6:1 were 67.6% and 49.3%, respectively.
In order to identify the rate-controlling step, extraction kinetics of zinc oxide and silica were analyzed using two different models. Based on experimental data in Fig. 3, the relationships of right-hand sides of Eq. (1) and Eq. (2) against reaction time are shown in Figs. 4 and 5, respectively. The apparent reaction rate constants kc and kp were obtained from the slopes of those straight fitting lines and showed along with their corresponding correlation coefficients R2 in Table 4. It was obvious that the correlation coefficients R2 for straight fitting lines in Fig. 5 were closer to 1 than those in Fig. 4. Similarly, the fitting lines in Fig. 5 were nearer to point 0 than those in Fig. 4.

Fig. 3 Influence of NaOH to zinc oxide ore molar ratio on extraction ratios of zinc oxide (a) and silica (b)

Fig. 4 Plots of 1-(1-α)1/3 against reaction time at different molar ratios:
Table 4 Apparent rate constants and correlation coefficients at different molar ratios


Fig. 5 Plots of 1-(1-α)2/3–2α/3 against reaction time at different molar ratios:
3.4.2 Effect of roasting temperature
The effect of roasting temperature on the extraction ratios of zinc oxide and silica was investigated from 350 °C to 500 °C. The molar ratio of NaOH to zinc oxide ore was 6:1. The experimental results are plotted in Fig. 6.
From Fig. 6, the roasting temperature played a significant role in extracting of zinc oxide and silica. The extraction ratios of zinc oxide and silica increased with the rising of roasting temperature. About 67% zinc oxide and 61% silica were extracted when roasting temperature was 500 °C and reaction time was 120 min. The extraction kinetics of zinc oxide and silica was investigated too. The plots of right-hand sides of Eq. (1) and Eq. (2) against reaction time are shown in Figs. 7 and 8, respectively.
The apparent rate constants kc and kp were obtained from the slops of those straight fitting lines in Figs. 7 and 8. The values of kc and kp as well as their corresponding correlation coefficients R2 are recorded in Table 5. All the straight fitting lines in Fig. 8 were nearer to zero point than those in Fig. 7. It seemed that the roasting reaction rate of zinc oxide ore using NaOH was controlled by the diffusion through product layer, which was consistent with the above.
In kinetic experiments, the muffle furnace was heated to the setting temperature first, then a series of crucibles with reactant mixture were put simultaneously and quickly into the furnace. Due to the limitation of experimental conditions, when the roasting temperature was stable again, the chemical reaction between zinc oxide ore and NaOH must have been going on, so the real extraction ratios of ZnO and SiO2 should be greater than zero when starting to time. It was essential to verify the rationality of experimental results by calculating the apparent activation energy E using Eq. (1) and Eq. (2).

Fig. 6 Effect of roasting temperature on extraction ratios of zinc oxide (a) and silica (b)
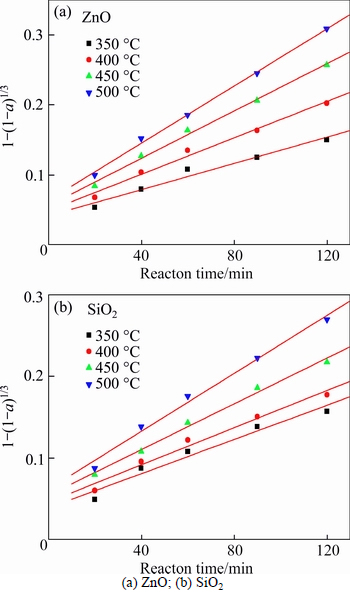
Fig. 7 Plots of 1-(1-α)1/3 against reaction time at different roasting temperatures:
3.4.3 Calculation of apparent activation energy
The reaction rate constant k is a temperature function, which can be expressed by Arrhenius equation [24]:
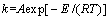 (3)
(3)
where A is frequency factor; E is apparent activation energy.
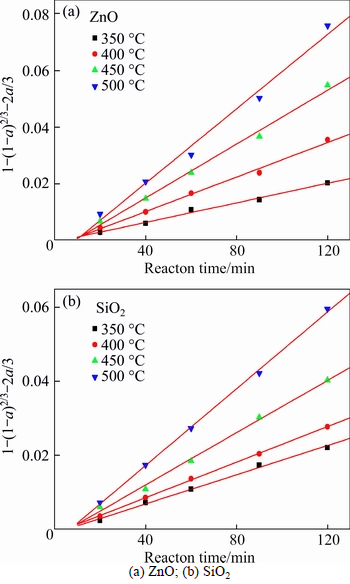
Fig. 8 Plots of 1-(1-α)2/3–2α/3 against reaction time at different roasting temperatures:
According to Eqs. (1) and (2), relationships of lnk to 1/T for ZnO and SiO2 are plotted in Figs. 9 and 10, respectively.
According to Figs. 9(a) and (b), the apparent activation energies (E) of ZnO’s and SiO2’s extracting reaction were calculated to be 20.81 kJ/mol and 13.97 kJ/mol, respectively. The corresponding frequency factors (A) were 0.0529 and 0.0148, respectively. The average E and average A were 17.39 kJ/mol and 0.0338, respectively.
Table 5 Apparent rate constants and correlation coefficients at different roasting temperatures
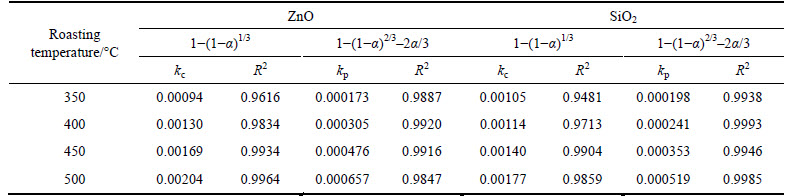
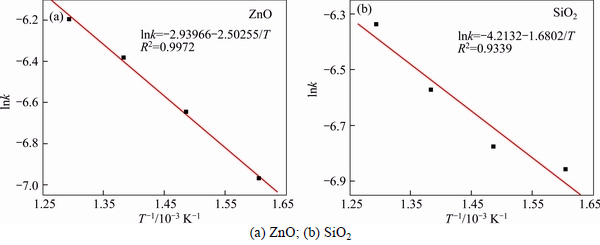
Fig. 9 Plots of lnk against 1/T at different roasting temperatures using Eq. (1):

Fig. 10 Plots of lnk against 1/T at different roasting temperatures using Eq. (2):
The apparent activation energies (E) calculated according to Figs. 10(a) and (b) were 35.79 kJ/mol and 25.90 kJ/mol, respectively. The corresponding frequency factors (A) were 0.1779 and 0.0273, respectively. The average E and average A were 30.85 kJ/mol and 0.1026, respectively. So, we can obtain the average apparent activation energy E of two calculation methods for roasting process was 24.12 kJ/mol.
Generally, when activation energy is lower than 20 kJ/mol, the chemical reaction meets diffusion control model. When activation energy is higher than 40 kJ/mol, the reaction meets chemical reaction control model [25, 26]. At the same time, when reaction activation energy is between 12 and 41.8 kJ/mol, the chemical reaction process should meet mixed control model [27]. So, the following equation is established.
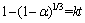 (4)
(4)
That is to say, in Eq. (4), 1–(1–a)1/3 and reaction time show a linear relationship, where a is reaction rate, k is average reaction rate constant, k=k1×k2/(k1+k2), k1 is reaction rate constant in chemical reaction control model, and k2 is reaction rate constant in diffusion control model. When k1>k2, the chemical reaction control is dominant; When k1<>2, the diffusion control is dominant.
In summary, the following kinetic equation is established.
 (5)
(5)
3.5 Characterization of residue after zinc extraction
Figures 11(a) and (b) show the XRD pattern and SEM image of water-leaching residue. The roasting reaction conditions are the appropriate reaction conditions obtained through orthogonal test.
The main phases in residue were quartz silica, Na2ZnSiO4, Ca2PbO4, Ca2SiO4, NaFeO2 and iron oxides. Due to the existences of Na2ZnSiO4, Ca2PbO4 and Ca2SiO4 which are very stable in alkaline condition, the extraction ratios of zinc oxide and silica were not high.
Table 6 lists the main chemical compositions in residue. The contents of Fe2O3, ZnO, SiO2, CaO and PbO2 were 47.92%, 10.29%, 12.95%, 14.23% and 1.94%, respectively. In combination with data in Table 1 and Table 6, the extraction ratios of zinc oxide and silica were about 83.20% and 71.21%, respectively, which were approximate to the results of orthogonal test. Fe2O3 in residue is valuable and can be retrieved too.

Fig. 11 XRD pattern (a) and SEM image (b) of residue
Table 6 Main compositions of residue

4 Conclusions
1) The optimized reaction conditions of extracting zinc oxide and silica were as follows: molar ratio of NaOH to zinc oxide ore 6:1, roasting temperature 450 °C, holding time 150 min. The molar ratio of NaOH to zinc oxide ore was the most predominant factor to influence the extraction ratios of zinc oxide and silica. The extraction ratios of zinc oxide and silica were about 83% and 72%, respectively.
2) The reaction kinetics equation is obtained, 
3) The amorphous silica reacted with molten NaOH to form Na2SiO3 at first, and then transformed into Na4SiO4 with the rising of roasting temperature, but quartz SiO2 did not react with NaOH. ZnCO3/ZnO reacted with NaOH to form Na2ZnO2 with temperature rising, and Na2ZnSiO4 was also synthesized.
References
[1] LI Yong, WANG Ji-kun, WEI Chang, LIU Chun-xia, JIANG Ji-bo, WANG Fan. Sulfidation roasting of low grade lead-zinc oxide ore with elemental sulfur [J]. Minerals Engineering, 2010, 23(7): 563–566.
[2] SUN Yi, SHEN Xiao-yi, ZHAI Yu-chun. Thermodynamics and kinetics of extracting zinc from zinc oxide ore by ammonium sulfate roasting method [J]. Int J Miner Metall Mater, 2015, 22(5): 467–475.
[3] ZHAO Chang-ming, ZHAI Yu-chun, ZHANG Chong-min, LI Jun-li, LI Sheng-li. Reaction mechanism of molten NaoH decomposing Zn2SiO4 in willemite [J]. Journal of Central South University, 2015, 22(4): 1227–1231.
[4] YIN Zhou-lan, DING Zhi-ying, HU Hui-ping, LIU Kui, CHEN Qi-yuan. Dissolution of zinc silicate (hemimorphite) with ammonia- ammonium chloride solution [J]. Hydrometallurgy, 2010, 103(1–4): 215–220.
[5] SHAO Hong-wei, SHZN Xiao-yi, SUN Yi, LIU Yan, ZHAI Yu-chun. Reaction condition optimization and kinetic investigation of roasting zinc oxide ore using (NH4)2SO4 [J]. International Journal of Mineral Metallurgy and Materials, 2016, 23(10): 1–8.
[6] CHEN Bing, SHEN Xiao-yi, GU Hui-min, SUN Yi, LI De-guan, ZHAI Yu-chun, MA Pei-hua. Extraction of ZnO from zinc oxide ore by alkali roasting method [J]. CIESC J, 2012, 63(2): 658–661. (in Chinese)
[7] SHEN Xiao-yi, SUN Yi, SONG Ji-qiang, ZHAI Yu-chun. Low grade zinc ore by low temperature roasting using (NH4)2SO4 [J]. Chin J Mater Res, 2012, 26(4): 396–401. (in Chinese)
[8] DOU Ai-chun, YANG Tian-zu, YANG Ji-xing, WU Jiang-hua, WANG An. Leaching of low grade zinc oxide ores in Ida2--H2O system [J]. Transactions of Nonferrous Metals Society of China, 2011, 21(11): 2548–2553.
[9] LI Cun-xiong, XU Hong-sheng, DENG Zhi-gan, LI Xing-bin, LI Ming-tin, WEI Chang. Pressure leaching of zinc silicate ore in sulfuric acid medium [J]. Transactions of Nonferrous Metals Society of China, 2010, 20(5): 918–923.
[10] HE Shan-ming, WANG Ji-kun, YAN Jiang-feng. Pressure leaching of synthetic zinc silicate in sulfuric acid medium [J]. Hydrometallurgy, 2011, 108(3, 4): 171–176.
[11] ESPIARI S, RASHCHI F, SADRNEZHAAD S K. Hydrometallurgical treatment of tailings with high zinc content [J]. Hydrometallurgy, 2006, 82(1, 2): 54–62.
[12] ABKHOSHK E, JORJANI E, AL-HARAHSHEH M S, RASHCHI F, NAAZERI M. Review of the hydrometallurgical processing of non-sulfide zinc ores [J]. Hydrometallurgy, 2014, 149(10): 153–167.
[13] WANG Zhi-fa, PENG Zhi-hui. Characteristic of thermometallurgy of zinc oxide ore [J] Journal of Jishou University, 1992, 13(6): 116–118. (in Chinese)
[14] MORADI S, MONHEMIUS A J. Mixed sulphide oxide lead and zinc ores problems and solutions [J]. Mineral Engineering, 2011, 24(10): 1062–1076.
[15] SHAO Hong-mei, SHEN Xiao-yi, ZHU Hui-ting, ZHAI Yu-chun. Process and reaction mechanism of roasting low grade zine oxide ore with (NH4)2SO4 [J]. The Chinese Journal of Nonferrous Metals, 2010, 20(6): 1148–1153. (in Chinese)
[16] YANG Tian-zu, DOU Ai-chun, LEI Cun-mao, REN Jin, LIU Zhen-zhen. Ligand selection for complex-leaching valuable metals in hydrometallurgy [J]. Transactions of Nonferrous Metals Society of China, 2010, 20(6): 1148–1153.
[17]  E, KOCAKERIM M M. Optimization study of the leaching of roasted zinc sulphide concentrate with sulphuric acid solutions [J]. Chemical Engineering and Processing, 2004, 43(8): 1007–1014.
E, KOCAKERIM M M. Optimization study of the leaching of roasted zinc sulphide concentrate with sulphuric acid solutions [J]. Chemical Engineering and Processing, 2004, 43(8): 1007–1014.
[18] CHEN Ai-liang, ZHAO Zhong-wei, JIA Xi-jun, LONG Shuang, HUO Guang-sheng, CHEN Xing-yu. Alkaline leaching Zn and its concomitant metals from refractory hemimorphite zinc oxide ore [J]. Hydrometallurgy, 2009, 97(3, 4): 228–232.
[19] FENG Lin-yong, YANG Xian-wan, SHEN Qing-feng, XU Ming-li, JIN Bing-jie. Pelletizing and alkaline leaching of powdery low grade zinc oxide ores [J]. Hydrometallurgy, 2007, 89(3, 4): 305–310.
[20] CHEN Qi-yuan, LI Liang, BAI Lan, HU Hui-ping, LI Jian, LIANG Qi-wen, LING Jiang-hua. Synergistic extraction of zinc from ammoniacal ammonia sulfate solution by a mixture of a sterically hindered beta-diketone and tri-n-octylphosphine oxide (TOPO) [J]. Hydrometallurgy, 2011, 105(34): 201–206.
[21] YANG Sheng-hai, TANG Mo-tang. Thermodynamics of Zn(Ⅱ)- NH3-NH4Cl-H2O system [J]. Transactions of Nonferrous Metals Society China, 2000, 10(6): 830–833.
[22] CHEN Bing, SHEN Xiao-yi, GU Hui-min, SHAO Hong-mei, ZHAI Yu-chun, MA Pei-hua. Comprehensive utilization of low-grade zinc oxide ore by alkaline molten roasting [J]. Multipurpose Utilization of Mineral Resources, 2016(5): 30–33. (in Chinese)
[23] LI Hong-gui. Metallurgical principles [M]. Beijing: Science Press, 2005. (in Chinese)
[24] HUA Yi-xin. Introduction of metallurgical process kinetics [M]. Beijing: Metallurgical Industry Press, 2004. (in Chinese)
[25] SHON H Y, WADSWORTH M E. Rate process of extractive metallurgy [M]. New York: Springer, 1979.
[26] CHEN Jia-yong. Handbook of hydrometallurgy [M]. Beijing: Metallurgical Industry Press, 2005. (in Chinese)
[27] MA Rong-jun. Principle on hydrometallurgy [M]. Beijing: Metallurgical Industry Press, 2007. (in Chinese)
(Edited by YANG Hua)
Cite this article as:
CHEN Bing, SHEN Xiao-yi, GU Hui-min, SHAO Hong-mei, ZHAI Yu-chun, MA Pei-hua. Extracting reaction mechanism analysis of Zn and Si from zinc oxide ore by NaOH roasting method [J]. Journal of Central South University, 2017, 24(10): 2266–2274.
DOI:https://dx.doi.org/https://doi.org/10.1007/s11771-017-3637-zFoundation item: Projects(51774070, 51204054) supported by the National Natural Science Foundation of China; Project(N150204009) supported by the Ministry of Education Basic Scientific Research Business Expenses, China; Project(2007CB613603) supported by the National Basic Research Program of China
Received date: 2017-06-19; Accepted date: 2017-09-14
Corresponding author: SHEN Xiao-yi, Associate Professor, PhD; Tel: +86–24–83687731; E-mail: shenxy@smm.neu.edu.cn
Abstract: The orthogonal test was used to optimize the reaction conditions of roasting zinc oxide ore with NaOH aiming to comprehensively utilize zinc oxide ore. The optimized reaction conditions were molar ratio of NaOH to zinc oxide ore 6:1, roasting temperature 450 °C, holding time 150 min. The molar ratio of NaOH to zinc oxide ore was the most predominant factor affecting the extraction ratios of zinc oxide and silica. The mineral phase transformations were investigated by testing the phases of specimens obtained at different temperatures. The process was that silica reacted with molten NaOH to form Na2SiO3 at first, then transformed into Na4SiO4 with temperature rising. ZnCO3 and its decomposing product ZnO reacted with NaOH to form Na2ZnO2. Na2ZnSiO4 was also obtained. The reaction rate was investigated using unreacted shrinking core model. Two models used were chemical reaction at the particle surface and diffusion through the product layer. The results indicated that the reaction rate was combine-controlled by two models. The activation energy and frequency factor were obtained as 24.12 kJ/mol and 0.0682, respectively.


