
![]()

Trans. Nonferrous Met. Soc. China 22(2012) 1156-1160
Effect of Er substituting sites on upconversion luminescence of Er3+-doped BaTiO3 films
CHEN Lei, WEI Xian-hua, FU Xu
State Key Laboratory Cultivation Base for Nonmetal Composites and Functional Materials,
Southwest University of Science and Technology, Mianyang 621010, China
Received 22 June 2011; accepted 15 February 2012
Abstract:
Erbium-doped BaTiO3 films on LaNiO3/Si substrates were fabricated by sol-gel method. The crystalline structure, morphologies and upconversion (UC) luminescence properties of films were respectively investigated by X-ray diffraction (XRD), atomic force microcopy (AFM) and photoluminescence (PL). The results indicate that both of the microstructure and luminescence are found to be dependent on Er3+ substituting sites. The samples with A-site substitution have smaller lattice constants, larger grains and smoother surface than those with B-site substitution. The photoluminescence spectra show that both of the samples have two stronger green emission bands centered at 528 and 548 nm and a weak red emission band centered at 673 nm, which correspond to the relaxation of Er3+ from 2H11/2, 4S3/2, and 4F9/2 levels to the ground level 4I15/2, respectively. Compared with B-site doped films, A-site doped films have a stronger integrated intensity of green emissions and a weaker relative intensity of red emissions. The differences could be explained by the crystalline quality and cross relaxation (CR) process.
Key words:
Er3+ doping; BaTiO3 thin films; upconversion photoluminescence; sol-gel method;
1 Introduction
Ferroelectric thin films for integrated optics have received much attention due to their potential applications in second-harmonic generation, high speed modulators and gain devices. Barium titanate (BaTiO3) is a good thin film integrated optic host because of its superior electronic and electro-optic properties, compared with other ferroelectric materials. For example, it has high electro-optic coefficient (820 pm/V at 632.8 nm, higher than 30.8 pm/V of LiNbO3) [1,2], low half-wave voltage (310 V at 632.8 nm, lower than 2940 V of LiNbO3) [3], low-loss waveguides (≤(4 ±2) dB/cm) and high solid solubility of rare earth ions [3,4]. Er3+ as an active ion can meet the request used for upconversion (UC) phosphors, planar waveguide and structural probe [5-8]. A lot of work about UC properties has been reported on Er3+-doped BaTiO3 films [3, 4, 6, 9]. It is noted that both of the valence state and the radius of Er3+ ion are intermediate between those of Ba2+ ion and Ti4+ ion. As a result, Er3+ can occupy either A- or B-site depending on Ba/Ti mole ratio [8,10-12]. It is well-known that crystal field caused by structure symmetry of the host materials would result in different perturbation in terms of Er3+ inner shell transitions. Therefore, UC photoluminescence should be dependent on the excited-state dynamics of the Er3+ ions and their interactions with the host matrix [8,13]. Unfortunately, little attention has been paid to the effect of Er substituting sites on UC photoluminescence properties. Recently, the UC properties of Er3+-doped BaTiO3 ceramics were correlated with doping concentrations, phase structure and doping sites [8,14]. Furthermore, the emission from substituted Er3+ ions might be considered structural probe for the ferroelectrics. In this work, the influence of substitution site of Er3+-doped BaTiO3 films on their photoluminescence properties is investigated. Er3+-doped BaTiO3 thin films with different doping sites are deposited on LaNiO3/Si substrate by sol-gel method.
2 Experimental
Er3+-doped BaTiO3 films were prepared by a modified sol-gel method. According to the charge compensation mechanism, the charge neutrality should be maintained through the vacancy formation of barium and oxygen for those samples with A-site and B-site substitution of Er3+, respectively. So, the formulas should be respectively Ba1-3x/2ErxTiO3 and BaTi1-xErxO3-δ. Here the value of x is 0.03, which has been proved to show a strong UC photoluminescence [15]. The whole process of preparing the solution is described in Fig. 1. Barium acetate [Ba(Ac)2], titanium butoxide [Ti(C4H9O)4, Ti(O-Bu)4], erbium acetate [Er(Ac)3] were used as starting materials; acetly acetone [AcAc, C5H8O2], acetic acid (HAc) were used as solvents of Ti(O-Bu)4 and Ba(Ac)2 respectively. 2-methoxyethanol was used as solvent for the whole solution. The concentration of the solutions was adjusted to 0.4 mol/L through the addition of ethylene glycol. A light brown-yellow and transparent precursor appeared in the final sol. The LaNiO3/Si substrates were first cleaned with acetone and ethanol, later rinsed with deionized water in a sonic bath. Spin-coating of the solutions was performed at 500 r/min for 18 s and 4000 r/min for 30 s on LaNiO3/Si substrates. After coating, each layer was dried at 200℃ for 10 min and then baked at 400℃ for 10 min in air. This process was repeated until the desired thickness of about 500 nm was attained. Finally, the samples were annealed in oxygen at 750℃ for 2 h.

Fig. 1 Process of preparing Er3+-doped BaTiO3 solutions
Thermogravimetric analysis (TGA) and differential thermal analysis (DTA) of dried gel were carried out on METTLER TOLEDO (TGA/SDTA 851e) from the room temperature to 800℃ in an oxygen flux with a heating rate of 10℃/min. The crystal structure of the thin films was analyzed by an X-ray diffractometer (XRD, Philips X’pert MPD Pro) utilizing Cu Kα (wavelength: λ= 1.540 6 ?) radiation. Atomic force microscope (AFM, Seiko SPI 3800) was used to investigate the grain size and surface roughness. The photoluminescence spectra were characterized by the use of Edinburgh FLSP920 spectrofluorophotometer at room temperature. The excitation light for UC luminescence was a 980 nm laser diode with a power of 500 mW. The excitation beams were applied directly onto the film surface and the signals were collected in the front of the films.
3 Results and discussion
3.1 DTA and TG
Figure 2 shows the TGA, DrTGA (derivative of TGA) and DTA results of BaTiO3 dry gel. The first peak of DrTGA (the inset of Fig. 2) appears at about 100℃, indicating the loss of water in dry gel. Significant mass losses of about 1.6 mg and 2 mg are observed in temperature range of 100-300℃ and 300-450℃, respectively. These may be due to the decomposition and combustion process of the organic compounds, which are in agreement with the exothermic peaks in DTA curve. The two peaks at 600℃ and 700℃ corresponding to the mass loss of about 0.5 mg and 0.3 mg represent the complete decomposition of the organic compounds and the formation of crystallized BaTiO3, respectively. Therefore, the treatment temperatures of films are selected as 400℃ for the baking process and 750℃ for the final annealing process.

Fig. 2 Thermal analysis results of BaTiO3
3.2 X-ray diffraction patterns and atomic force microscopy image
Figure 3(a) presents the XRD patterns of Er3+-doped BaTiO3 thin films annealed at 750℃. Only the diffraction peaks from phase-pure crystalline BaTiO3 is observed besides the existence of the information of the substrates in both cases. The result implies that Er3+ ions were doped efficiently into different sites of BaTiO3 host. There are minor shifts of the diffraction peak (200) between the Er3+-doped samples, as shown in Fig. 3(b), meaning that the lattices of the films could be slightly deformed because of the doping of impurity ions. In two cases, no obvious splitting of cubic (200) into tetragonal (200) and (002) reflections at about 45° can be observed. It remains difficult to assign the crystal structure of BaTiO3 films to either cubic or tetragonal symmetry using conventional X-ray diffractometer due to line broadening [16]. Otherwise, it should be induced by the size effect. Here the BaTiO3 lattice was regarded as cubic phase with the lattice parameter of 3.9820 ? for the undoped sample (JCPS Card No.74—1956). The computed lattices are respectively 3.9745 ? and 3.9872 ? from the XRD results for the A-site and B-site doped films, indicating the contraction and expansion of the lattice [8,17].

Fig. 3 XRD patterns of films doped with 3% Er3+ for A- and B-site BaTiO3 substitution annealed at 750℃ (a) and enlarged (200) peak (b)
Moreover, the FWHM of B-site substitution sample is larger than that of A-site substitution one, indicative of the smaller grains. It can be further proved by AFM analysis shown in Fig. 4. The films of A-site substitution have a well-crystallized and dense surface with the average grain size of about 60 nm and the RMS roughness of 4.0 nm. On the other hand, the samples of B-site substitution show the smaller grain size of about 40 nm and the larger RMS value of 8.4 nm (not shown here). The difference of the crystalline quality should be associated with the oxygen-deficient nonstoichiometry in B-site doped films.
3.3 UC photoluminescence
The PL spectra of BaTiO3 films with different Er3+ ion site substitutions are measured under excitation of 980 nm, as shown in Fig. 5. The typical UC emission consists of two strong green bands located at 528 and 548 nm corresponding to 2H11/2/4S3/2→4I15/2 transitions, and a weak red emission band at 673 nm associated with 4F9/2→4I15/2 transition of the Er3+ ions, respectively. The intensity ratio of 528 and 548 nm (I548/I528) is about 1.35 for both of the cases, while the relative ratios of green and red (I548/I673) are respectively 9.25 and 4.83 for A-site substitution and B-site substitution. There is no obvious shift of the emission peaks. These results indicate that the crystal field surrounding the erbium ions can change the relative probability of the transitions from the excited state to the 4I15/2 ground state. The UC luminescence mechanisms are mainly determined by the process of excited state absorption (ESA) and energy transfer (ET). As shown in Fig. 6, under the excitation of 980 nm, through ESA or ET process, Er3+ ion can populate the 4F7/2 level. Subsequently, the Er3+ ion then relaxes nonradiatively to the 2H11/2, 4S3/2 and 4F9/2 levels by multiphonon relaxation, from which the strong green 2H11/2/4S3/2→4I15/2 emissions and the weak red 4F9/2→ 4I15/2 occur. The ESA approach only occurs during excitation, whereas the ET can happen both during and after the excitation. For the 673 nm red emission, due to the large energy gap between 4S3/2 and 4F9/2 levels, the nonradiative relaxation probability from the 4S3/2 level to the 4F9/2 level is quite low. Therefore, the intensity of the red emissions is weaker than that of green emissions [18].

Fig. 4 AFM micrograph of 3% Er3+-doped BaTiO3 film with A-site

Fig. 5 Integrated intensity of BaTiO3 doped with 3% Er3+ for A- and B-site substitutions
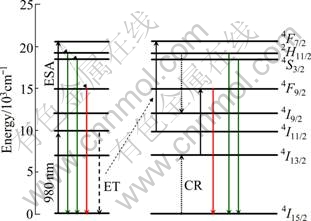
Fig. 6 Possible UC photoluminescence mechanisms of 3% Er3+-doped BaTiO3 films
The integrated intensity of A-site doped sample is much stronger than that of B-site doped BaTiO3 film. It is mainly affected by the crystalline condition which A-site substitution has a better crystalline. But there is no difference between them in the value of I548/I528, which indicates that the ratio of I548 to I528 has no great importance in different substituting positions and crystalline conditions. As for the large difference of I548/I673, it could be understood by cross-relaxation (CR) shown in Fig. 6. As we known, subband gap defect levels could participate in the relaxation process and change the probability of radiative recombination [8, 19, 20]. Compared with A-site sample, B-site substitution has higher defect density which could enhance ET probability of CR process, i.e. 2H11/2+4I15/2→4I9/2+4I13/2. Then the Er3+ ions at 4I13/2 state populate the 4F9/2 state through ESA, which enhances the relative intensity of the red emission [8]. Furthermore, the population of 4S3/2 state will decrease because a part of Er3+ ions populate the 2H11/2 state [21], which also weakens the integrate intensity of the green emission. Therefore, the green-to-red ratio of B-site doped sample decreases relatively to that of A-site substitution.
4 Conclusions
1) Er3+-doped BaTiO3 nano-luminescent films were fabricated on LaNiO3 substrate by sol-gel method. The films only have the diffraction peaks of BaTiO3 and the peaks (200) shift for Er3+ doped A-site or B-site film, which indicates that Er3+ ions are doped efficiently into different sites of BaTiO3 host.
2) B-site doped film has a worse crystalline than A-site substitution, which should be attributed to the oxygen-deficient nonstoichiometry in B-site doped films.
3) Strong green UC emissions at 528 and 548 nm were obtained under the excitation of a 980 nm laser diode. The different UC photoluminescence properties were observed for Er3+-doped samples with different lattice sites. A-site doped film has a stronger integrated intensity of green emissions and a weaker relative intensity of red emissions, which could be related to the better crystalline and the weaker CR process respectively.
Acknowledgements
The authors are grateful to Dr. J. H. HAO and Y. ZHANG (The Hong Kong Polytechnic University) for technical support and useful discussion during the photoluminescence measurements.
References
[1] BLOCK B A, WESSELS B W. Photoluminescence properties of Er3+-doped BaTiO3 thin films [J]. Applied Physics Letters, 1994, 65(1): 25-27.
[2] GILL D M, FORD G M, BLOCK B A, KIM S S, WESSELS B W, HO S T. Guided wave absorption and fluorescence in epitaxial Er:BaTiO3on MgO [J]. Thin Solid Films, 2000, 365(1): 126-128.
[3] YANG Xu-dong, GUO Hai, CHEN Kun, ZHANG Wei-ping, LOU Li-ren, YIN Ming. Er3+ doped BaTiO3 optical-waveguide thin films elaborated by sol-gel method [J]. Journal of Rare Earth, 2004, 22(1): 36-39. (in Chinese)
[4] GILL D M, BLOCK A, CONRAD C W, WESSELS B W, HO S T. Thin film channel waveguides fabricated in metal-organic chemical vapor deposition grown BaTiO3 on MgO [J]. Applied Physics Letters, 1996, 69(20): 2968-2970.
[5] LIU Y X, PISARRDKI W A, ZENG S J, XU C F, YANG Q B. Tri-color upconversion luminescence of rare earth doped BaTiO3 nanocrystals and lowered color separation [J]. Optics Express, 2009, 17(11): 9089-9098.
[6] SCHLAGHECKEN G, GOTTMAN J, KREUTZ E W, POPRAWE R. Pulsed laser deposition of Er:BaTiO3 for planar waveguides [J] Applied Physics A, 2004, 79: 1255-1257.
[7] POLMAN A. Erbium as a probe for everything? [J] Physica B, 2001, 300: 78-90.
[8] ZHANG Y, HAO J H, MAK C L, WEI X H. Effects of site substitutions and concentration on Up-conversion luminescence of Er3+-doped perovskite titanate [J]. Optics Express, 2011, 19(3): 1824-1829
[9] ZHANG H X, KAM C H, ZHOU Y, BUDDHUDU S, XIANG Q, LAM Y L, CHAN Y C. Green up-conversion luminescence in Er3+:BaTiO3 films [J]. Applied Physics Letters, 2000, 77(5): 609-611.
[10] DUNBAR T D, WARREN W L, TUTTLE B A, RANDALL C A, TSUR Y. Electro paramagnetic resonance investigations of lanthanide-doped barium titanate:dopant site occupancy [J]. Journal of Physics Chemistry B, 2004, 108(3): 908-917.
[11] TAKSDA K, CHANG E, SMYTH D M. Rare-earth addition to BaTiO3 [J]. Advanced Ceramics, 1987, 19: 147-152.
[12] MITIC V V, NIKOLIC Z S, PAVLOVIC V B, PAUNOVIC V, MILJKOVIC M. Influence of rare-earth dopants on barium titanate ceramics microstructure and corresponding electrical properties [J]. Journal of American Ceramics Society, 2010, 93(1): 132-137.
[13] WEN C H, CHU S Y, JUANG Y D, WEN C K. New phase transition of erbium-doped KNbO3 polycrystalline [J]. Journal of Crystal Growth, 2005, 280(1-2): 179-184.
[14] CHEN L, LIANG X L, LONG Z, WEI X H. Upconversion photoluminescence properties of Er3+-doped BaxSr1-xTiO3 powders with different phase structure [J]. Journal of Alloys and Compounds, 2012, 516: 49-52.
[15] ZHANG H X, KAM C H, ZHOU Y, HAN X Q, XIANG Q, BUDDHUDU S, LAM Y L, CHAN Y C. Photoluminescence at 1.54 mm in sol-gel-derived, Er-doped BaTiO3 films [J]. Journal of Alloys Compounds, 2000, 308: 134-138.
[16] HUANG L M, CHEN Z Y, WILSON J D, BANERJEE S, ROBINSON R D, HERMAN I P, LAIBOWITZ R, BRIEN S O. Barium titanate nanocrystals and nanocrystal thin films: Synthesis, ferroelectricity and dielectric properties [J]. Journal of Applied Physics, 2006, 100: 034316.
[17] JIANG C G, FANG L, SHEN M R, ZHENG F G, WU X L. Effects of Eu substituting positions and concentrations on luminescent, dielectric, and magnetic properties of SrTiO3 ceramics [J]. Applied Physics Letters, 2009, 94: 071110.
[18] GAO F, WU G H, ZHOU H, BAO D H. Strong up-conversion luminescence properties of Yb3+ and Er3+ co-doped Bi4Ti3O12 ferroelectric thin films [J]. Journal of Applied Physics, 2009, 106:126104.
[19] HAO J H, STUDENIKIN S A, COCIVERA M. Transient photoconductivity properties of tungsten oxide thin prepared by spray pyrolysis [J]. Journal of Applied Physics, 2001, 90(10): 5064- 5069.
[20] WANG Z L, CHAN H L W, LI H L, HAO J H. Highly efficient low-voltage cathodoluminescence of LaF3:Ln3+ (Ln=Eu3+, Ce3+, Tb3+) spherical particles [J]. Applied Physics Letters, 2008, 93(14): 141106.
[21] OKAMOTO E, SEKITA M, MASUI H. Energy transfer between Er3+ ions in LaF3 [J]. Physical Review B, 1975, 11: 5103-5111.
Er3+掺杂位置对Er3+:BaTiO3薄膜上转换发光的影响
陈 磊,魏贤华,傅 旭
西南科技大学 四川省非金属复合与功能材料重点实验室-省部共建国家重点实验室培育基地,绵阳621010
摘 要:采用溶胶-凝胶法在LaNiO3/Si衬底上制备Er3+掺杂BaTiO3薄膜。通过XRD、AFM和PL图谱分别研究薄膜的晶体结构、形貌以及上转换发光性能。结果表明,薄膜的微观结构和发光性能与Er3+掺杂晶格的位置有关。A位掺杂薄膜较B位掺杂薄膜具有较小的晶格常数和较好的结晶。PL光谱表明:A位掺杂的薄膜和B位掺杂的薄膜都于528 nm和548 nm处获得较强的绿色上转换发光以及在673 nm处获得较弱的红光,分别对应Er3+离子的2H11/2→4I15/2,4S3/2→4I15/2和4F9/2→4I15/2能级跃迁。相对于B位掺杂的薄膜,A位掺杂样品有较强的绿光发射积分强度以及较弱的红光发射相对强度。这种差异可以通过薄膜的结晶状况和交叉弛豫机制来进行解释。
关键词:Er3+掺杂;BaTiO3薄膜;上转换发光;溶胶-凝胶法
(Edited by YANG Hua)
Foundation item: Project (2009AA035002) supported by the High-tech Research and Development Program of China
Corresponding author: WEI Xian-hua; Tel: +86-816-2419201; E-mail: weisansao@yahoo.com.cn
DOI: 10.1016/S1003-6326(11)61299-5
Abstract: Erbium-doped BaTiO3 films on LaNiO3/Si substrates were fabricated by sol-gel method. The crystalline structure, morphologies and upconversion (UC) luminescence properties of films were respectively investigated by X-ray diffraction (XRD), atomic force microcopy (AFM) and photoluminescence (PL). The results indicate that both of the microstructure and luminescence are found to be dependent on Er3+ substituting sites. The samples with A-site substitution have smaller lattice constants, larger grains and smoother surface than those with B-site substitution. The photoluminescence spectra show that both of the samples have two stronger green emission bands centered at 528 and 548 nm and a weak red emission band centered at 673 nm, which correspond to the relaxation of Er3+ from 2H11/2, 4S3/2, and 4F9/2 levels to the ground level 4I15/2, respectively. Compared with B-site doped films, A-site doped films have a stronger integrated intensity of green emissions and a weaker relative intensity of red emissions. The differences could be explained by the crystalline quality and cross relaxation (CR) process.


