Article ID: 1003-6326(2005)05-0993-04
Interface structure and formation mechanism of vacuum-free vibration liquid phase diffusion-bonded joints of SiCp/ZL101A composites
YAN Jiu-chun(闫久春), XU Hui-bin(许惠斌),
XU Zhi-wu(许志武), LI Da-cheng(李大成), YANG Shi-qin(杨士勤)
(State Key Laboratory of Advanced Welding Production Technology, Harbin Institute of Technology, Harbin 150001, China)
Abstract:
The vacuum-free vibration liquid phase(VLP) diffusion-bonding of SiCp/ZL101A composites was investigated. The effects of vibration on the interface structure, the phase transformation and the tensile strength of bonded joints were examined. Experimental results show that the oxide film on the surface of the composites is a key factor affecting the tensile strength of boned joints. The distribution of the oxide layers at the interface changes from a continuous line to a discontinuous one during vibration. The tensile strength of the VLP diffusion-bonded joints increases with the vibration time, and is up to the maximum of 172MPa when the vibration time is 30s. The phase structure of the bond region changes from the Zn-Al-Cu hyper-eutectic (η+(β+η)+(β+η+ε)) phases to Al-rich Al-base solid solution (α-Al) with increasing the vibration time.
Key words:
SiCp/ZL101A; diffusion bonding; vibration; interface structure; oxide film CLC number: TG453;
Document code: A
1 INTRODUCTION
Particulate reinforced aluminum metal matrix composites(MMCs) have been widely applied due to their excellent properties, and the welding technology associated is increasingly emphasized[1-3]. Many approaches such as brazing[4-6], diffusion bonding[7-9] and transient liquid phase bonding[10-14] have been used to bond the materials. However, their applications have been severely restricted by time-consuming and expensive operation. To overcome those problems, the vacuum-free bonding processes are increasingly paid attention to. Zuruzi et al[15, 16] used an interface rotation treatment technique for removing the oxide film on the surface to realize vacuum-free diffusion bonding of 6061Al. Lee et al[17] employed an in-situ surface treatment to remove the oxide film during vacuum-free diffusion bonding of Al-MMCs. Yokota and Otauko[18] made a specimen with a conical end to rotate for removing the oxide film on the surface of SiCp/6061Al. So far, literatures about bonding of aluminum alloy and Al-MMCs with the aid of vibration technique havent been reported. In this study, the SiCp/ZL101A composites were bonded by the vacuum-free vibration liquid phase bonding with Zn-Al filler metal. The effects of vibration on the interface structure, the phase transformation and the tensile strength of bonded joints were investigated. The formation mechanism of the bonded joints was discussed during vacuum-free VLP bonding.
2 EXPERIMENTAL
The material used in this work, made by stirring casting, contained 20%(volume fraction) SiC particles with ZL101A aluminum alloy. The samples were cylindrical rods with a size of d10mm×40mm. The filler metal was the Zn-Al-Cu hyper-eutectic (η+(β+η)+(β+η+ε)) alloy, its chemical composition and liquidus-solidus temperature were Zn-89.3%, Al-4.2%, Cu-3.22%, Mg-0.82%, Mn-0.91%, Si-0.81% and 399-383℃, respectively. The used filler metal was with a size of d10mm×0.5mm.
The vibration with an amplitude of 50μm and a frequency of 50Hz was applied under a pressure of 0.5MPa when the samples were heated to 530℃ by a high frequency induction coil, and then the bonding temperature kept constant for 2min under the pressure of 4MPa after the vibration stopped.
The microstructures of bonded joints were examined by energy dispersive X-ray spectrometer (EDX, TN5500) and electron probe X-ray microanalyser(EPMA, JEOL-733). The tensile strength of the bonded joints was evaluated by means of electron tension testing machine (Instron-5569).
3 RESULTS AND DISCUSSION
Fig.1 shows the effect of the vibration time on the tensile strength of the bonded joints. It can be seen that the tensile strength increases rapidly with the increasing vibration time when the vibration time is less than 20s. When the vibration time extends over 20s, the tensile strength increases slightly with the increasing vibration time, and the tensile strength of joint is up to the maximum(172MPa) when the vibration time is 30s. Then, the tensile strength of joints starts to decrease when the vibration time continues to increase.
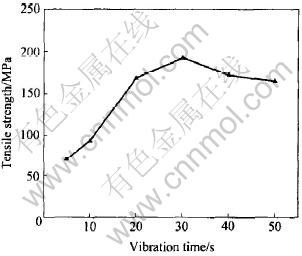
Fig.1 Tensile strengths of joints bonded at 530℃ for different vibration times
Fig.2 shows the back-scattered electron images of the cross-section of the joint bonded for no vibration and joint vibration-bonded for 5s and 30s. Fig.2(a) shows the back-scattered electron image of the cross-section of the joint bonded for no vibration at 530℃. It can be found from the figure that the three kinds of different microstructure zones have clearly occurred in the joint, which are marked by A zone, B zone and C zone, respectively. In addition, three kinds of smaller microstructure zones in A zone are observed, and marked by A1, A2 and A3 zone, respectively.
It can be inferred that A1, A2, A3 zone are composed of Zn-based solid solution (primary η phase), Al-based Zn-Al binary eutectic (η phase and β phase), and Zn-Al-Cu ternary eutectic (η phase, β phase and ε phase), respectively, according to the results examined by EDX (listed in Table 1). So A zone (the filler metal zone) is composed of η+(β+η)+ (β+η+ε). Similarly, according to Table 1, B zone is composed of the continuous oxide inclusion zone of Al, Zn and Cu at the interface, and C zone is composed of Zn-rich Al-based solid solution. In brief, the interface structure of joints bonded without vibration characterizes a hyper-eutectic alloy of 30-40μm, the continuous oxide inclusion zones, and the penetration zones of Zn and Cu in aluminum matrix with the width of 15-20μm.
Table 1 Chemical composition of each zone in joint VLP diffusion-bonded
for no vibration at 530℃(mole fraction, %)
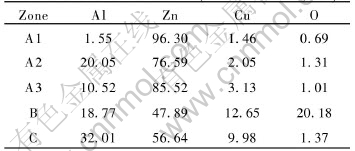
Fig.2(b) shows the back-scattered electron image of the cross-section of the joint vibration-bonded for 5s at 530℃. It can be found from the figure that the bonded joint is also composed of three kinds of different microstructure zone (zone A, zone B and zone C). Moreover, zone A is composed of two smaller microstructure zone which marked by A1 and A2 respectively, and a few of SiC particles segregated from the dissolved matrix.
Similarly, according to the results examined by EDX (shown in Table 2), it can be inferred that zone A is Zn-rich Al-based solid solution zone (A1), Al-rich Al-based solid solution zone (A2) and a few of SiC particles segregated from the dissolved matrix. zone B is also mainly composed of the discontinuous oxide inclusion zone of Al, Zn and Cu in the interface. zone C is composed of α phase (i.e. Al-rich Al-base solid solution).
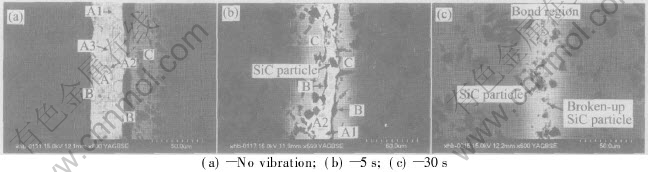
Fig.2 Back-scattered electron images of cross-section of joint vibration-bonded at 530℃
Table 2 Chemical composition of each zone in the joint VLP diffusion-bonded
for 5s at 530℃ (mole fraction, %)
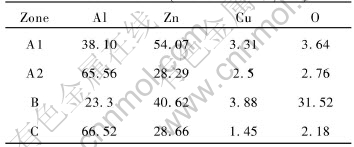
Consequently, the interface structure of joint vibration-bonded for 5s characterizes a mixture of Zn-rich and Al-rich Al-base solid solution of 10-15μm with a few SiC particles segregated from the matrix, the discontinuous oxide inclusion zones, and the penetration zones of Zn and Cu in aluminum matrix with the width of 15-20μm. The bonded joints have some strength because of partially metallurgic bonding between the filler metal and the base metal (see Fig.1).
It can be found from Fig.2(c) that the interface structure of joint vibration-bonded for 30s is very different from that of joint bonded for no vibration and joint vibration-bonded for 5s. The vibration-bonded joints for 30s are only composed of a gray bond region of 15-20μm distributed with some SiC particles. Some of those SiC particles in the bond region are larger ones, which directly segregate from the dissolved base metal; and the others are smaller ones, which are produced by shearing action among the particles segregated during vibrating. Moreover, the oxide inclusion in the interface disappears at the time.
The chemical composition of the matrix in the region VLP-bonded for 30s at 530℃ by EDX is Al-74.90%, Zn-22.06%, Cu-1.64%, O-1.39%(mole fraction). It can be inferred that the matrix zone of the joint is composed of Al-rich Al-based solid solution (α phase). Thus, the bond region is composed of SiC particle reinforced aluminum matrix composites, and the joints should show a high tensile strength (see Fig.1).
On basis of the above analysis results, it can be found that the remarkable changes of the microstructure occur with the increasing vibration time. The layer of the oxide inclusion becomes narrow and discontinuous. At the same time, the metallurgic bond between the aluminum matrix and the filler metal enhance with the decrease of oxide layer. The phase structure of the interlayer undergoes a remarkable change: from Zn-Al-Cu hyper-eutectic (η+(β+η)+(β+η+ε)) phases into Al-rich Al-based solid solution (α phase) after vibration is applied for 30s.
To describe the changing process of the interface structure of bonded joints, an evolution model was developed for VLP bonding of SiCp/ZL101A on basis of the above observations and experimental results. The model is shown in Fig.3. In the first stage, the oxide inclusion layers on the surface of the composites are disrupted, and the part of the liquid filler metal is squeezed out of joints (see Fig.3(b)). In the second stage, as the amount of dissolved liquid layers formed in the base metal increases by the diffusion of Zn and Cu in base metal, more SiC particles segregated from the composites enter into the liquid layer (see Fig.3(c)). In the third stage, as the amount of the dissolved liquid metal do not increase at some time, the total amount of liquid metal starts to decrease at the aid of pressure and vibration (see Fig.3(d)). In the forth stage, the two sides of the base metal come on contact and clash at the centerline of the joints, the fine particles form in the bond region by shearing action among the particles segregated during vibrating (see Fig.3(e)). Finally, the particles in the solution layer segregate in the centerline of the bond region during isothermal solidification (see Fig.3(f)).
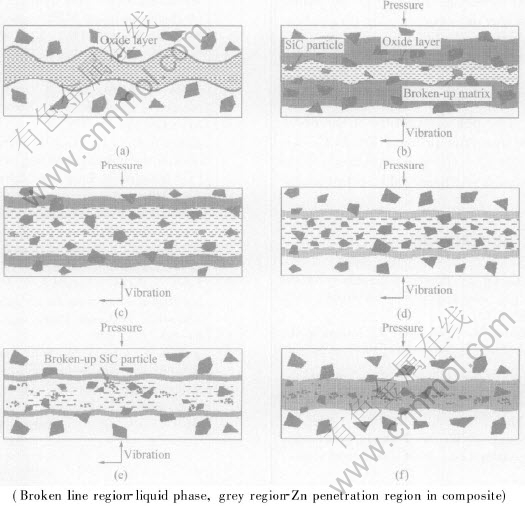
Fig.3 Interface structure evolution models for vibration liquid phase bonding of SiCp/ZL101A
4 CONCLUSIONS
1) Vibration was introduced to disrupt the oxide film on the surface of the SiCp/ZL101A composites during VLP bonding in air. The metallurgic bond between the Zn-Al filler metal and the matrix forms. The tensile strength of the bonded joints increases with the vibration time, and is up to the maximum of 172MPa when the vibration time is 30s, then decreases slightly as the vibration time continues to increase.
2) During VLP bonding, the interface structure of VLP bonded joints characterizes an aluminum-based solid solution with a few SiC particles, and the penetration zones of Zn and Cu in aluminum matrix. The phase structure of the bonded joints changes from the primary Zn-Al-Cu hyper-eutectic (η+(β+η)+(β+η+ε) phases) of the filler metal to Al-rich Al-base solid solution (α-Al).
REFERENCES
[1]Midling O T, Grong . Joining of particle reinforced Al-SiC MMCs [J]. Key Engineering Materials, 1995, 104-107: 355-372.
[2]Ellis M B D, Gittos M, Threadgill P L. Joining of aluminum base metal matrix composites [J]. International Materials Reviews, 1996, 42(2): 41-58.
[3]Klehn R, Eager T W. Joining of 6061 aluminum matrix-ceramic particle reinforced composites [J]. Welding Research Council Bulletin, 1993, 385: 1-26.
[4]Zhang X P, Quan G F, Wei W. Preliminary investigation on joining performance of SiCp-reinforced aluminium metal matrix composite (Al/SiCp-MMC) by vacuum brazing [J] Composites Part A, 1999, 30(6): 823-827.
[5]YAN Jiu-chun, XU Zhi-wu, L Shi-xiong, et al. Microstructure characteristics of interaction layer of Zn-Al eutectic alloy with Al2O3p/6061Al composites [J]. J Mater Sci Technol, 2004, 20(4): 401-404.
[6]XU Zhi-wu, YAN Jiu-chun, LU Shi-xiong, et al. Microstructure of interaction interface of Al-Si and Zn-Al alloys with Al2O3p/6061Al composites [J]. Trans Nonferrous Met Soc China, 2004, 14(2): 351-355.
[7]Enjo T, Ikeuchi K, Akikawa N. Effect of oxide film on the early process of diffusion welding [J]. Transactions of JWRI, 1981, 10(2): 45-53.
[8]Enjo T, Ikeuchi K, Murakami Y. Diffusion bonding of Al-Si-Mg series 6063 alloy reinforced with Al2O3 short fiber [J]. Transactions of JWRI , 1987, 16(2): 57-64.
[9]Zhao M J, Chen L Q, Bi J. Diffusion bonding of silicon carbide particulate reinforced 2024Al composites [J]. J Mater Sci Technol, 2000, 16(5): 471-474.
[10]Zhai Y, North T H. Transient liquid-phase bonding of aluminum and metal matrix composite base materials [J]. Journal of Materials Science, 1997, 32: 1393-1397.
[11]Askew J R, Wilde J F, Khan T I. Transient liquid phase bonding of 2124 aluminum metal matrix composite [J]. Materials Science and Technology, 1998, 14(9): 920-924.
[12]Shirzadi A A, Wallach E R. New approaches for transient liquid phase diffusion bonding of aluminium based metal matrix composites [J]. Materials Science and Technology, 1997, 13(2): 135-142.
[13]Li Z, Zhou Y, North T H. Counteraction of particulate segregation during transient liquid-phase bonding of aluminum-based MMC material [J]. Journal of Materials Science, 1995, 30: 1075-1082.
[14]Yan J C, Xu Z W, Wu G H, et al. Interface structure and mechanical performance of TLP bonded joints of Al2O3p/6061Al composites using Cu/Ni composite interlayers [J]. Scripta Materialia, 2004, 51: 147-150.
[15]Zuruzi A S, Li H, Dong G. Effects of surface roughness on the diffusion bonding of al alloy 6061 in air [J]. Materials Science and Engineering A, 1999, 270(2): 244-248.
[16]Zuruzi A S, Li H, Dong G. Diffusion bonding of aluminum alloy 6061 in air using an interface treatment technique [J]. Materials Science & Engineering A, 1999, 259(1): 145-148.
[17]Lee C S, Li H, Chandel R S. Vacuum-free diffusion bonding of aluminium metal matrix composite [J]. Journal of Materials Processing Technology, 1999, 89-90: 326-330.
[18]Yokota T, Otauka M. Solid phase welding of alloy AA6061 and SiCp reinforced alloy AA6061 at intermediate temperature [J]. Materials science Forum, 1997, 242: 225-230.
Foundation item: Project(50375039) supported by the National Natural Science Foundation of China
Received date: 2004-12-22; Accepted date:2005-04-18
Correspondence: YAN Jiu-chun, Professor, PhD; Tel: +86-451-86418695; Fax: +86-451-86416186; E-mail: jcyan@hope.hit.edu.cn


