Trans. Nonferrous Met. Soc. China 28(2018) 163-168
Selective flotation of smithsonite, quartz and calcite using alkyl diamine ether as collector
Hai-ling ZHU1,2, Wen-qing QIN2, Chen CHEN2, Li-yuan CHAI1, Lai-shun LI2, San-jun LIU2, Ting ZHANG2
1. School of Metallurgy and Environment, Central South University, Changsha 410083, China;
2. School of Minerals Processing and Bioengineering, Central South University, Changsha 410083, China
Received 22 September 2016; accepted 13 January 2017
Abstract:
The flotation separation of smithsonite from calcite and quartz using a alkyl diamine ether (GE-609) as the collector was investigated through micro-flotation experiments and the real ore flotation experiments. The results show that GE-609 exhibits good collecting capability to three minerals without selectivity. The presence of sodium sulfide enhances the flotation of smithsonite and calcite while inhibits quartz. Moreover, both sodium silicate and sodium hexametaphosphate exhibit good selective inhibition to calcite. The real ore test results show that a zinc concentrate containing 23.51% Zn with the recovery of 71.02% is obtained in the closed-circuit test. To understand the adsorption of GE-609 on smithsonite surface, zeta potential measurement and FTIR analysis were carried out, and the results indicate that the collector GE-609 can adsorb on smithsonite surface through both electrostatic adsorption and chemical adsorption, and the presence of sodium sulfide enhances the adsorption of GE-609.
Key words:
smithsonite; quartz; calcite; alkyl diamine ether; sodium sulfide; sulphidization flotation;
1 Introduction
Although there is a wealth of zinc oxide ore resources in China, the lean ores occupy the most part, with the continuously increasing of exploration difficulty, the comprehensive utilization of low-grade zinc ores has become a pressing task. Smithsonite (ZnCO3) is a typical oxidized zinc ore, and it usually appears in groups with calcite, quartz, dolomite and other gangue minerals. Therefore, it is required to achieve the separation in order to obtain a marketable product.
Flotation is a commonly used method for beneficiation of smithsonite [1-3], and two important methods have long been used. The one is direct flotation using fatty acids [4-6], chelating agents [7] or mercaptan [8] as collector, the other is sulphidization and then flotation with amines [9-11] or xanthates [12,13]. Although sodium silicate and sodium hexameta- phosphate were used as depressants, the grade and recovery of zinc with oleic acid as the collector were lower than those obtained using the sulphidization- cationic flotation method [14]. In case of commercially available chelating agents, it is difficult to achieve large-scale application due to the lack of a long-chain hydrocarbon in the molecule [7]. For hexylmercaptan, the consumption is high [15]. Compared with sulphidization–flotation with xanthates, the technology of sulphidization–flotation with amines has been proved to be more effective in a large number of industrial practices, since it has advantages in sulphidization at normal temperature, without activation, and high selectivity.
The reagents used for sulphidizing the mineral mainly contain sodium sulphide, sodium hydrogen sulphide, elemental sulfur and sodium polysulfide. Sodium sulphide is cheaper than sodium hydrogen sulphide and also generates a high pH value [8,16]. The primary amines containing 8-18 carbon atoms exhibit strong collecting capability and selectivity to smithsonite after sulphidizing with sodium sulphide because they can react with copper and zinc to form complexes, but cannot react with calcium and magnesium [17,18]. However, the flotation is dependent on pH value [17], and amines show high sensitivity and poor selectivity for slimes which can deteriorate the flotation results [19].
Ether amine is obtained by introducing ether group into the hydrocarbon of an aliphatic amine, so it is easier to disperse in the pulp due to the lower melting point and higher solubility. GE-609 is a kind of alkyl diamine ether, and it has been successfully applied in the flotation practice of iron ore [20,21]. Compared with the common dodecylamine, GE-609 has better collecting capability and selectivity at the similar dosage. Furthermore, the use of GE-609 is more beneficial for actual production due to the less flotation foam with better brittleness. However, little effort has been devoted to the process and mechanism of smithsonite flotation with an ether amine as the collector. In this work, the main factors involved in the separation of smithsonite from calcite and quartz were investigated by micro-flotation experiments using GE-609 as the collector. And the adsorption of GE-609 on smithsonite surface was analyzed by zeta-potential measurement and Fourier transform infrared (FTIR) spectroscopy.
2 Experimental
2.1 Materials and reagents
The smithsonite sample was obtained from Yunnan province, China, and the calcite and quartz samples were obtained from Hunan province of China. They were crushed, ground and then sieved to collect the size fraction of 38-74 μm for the micro-flotation experiments. Based on the results of X-ray diffraction (XRD) and chemical analysis, the purities of the three samples are above 90%.
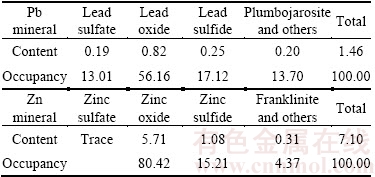
The real ore was obtained from Yunnan province, China. The chemical analysis results show that the raw sample contains 1.44% Pb, 7.04% Zn, 1.89% S, 4.72% Fe, 32.43% SiO2, 18.62% CaO, and so on. Based on XRD detection, the main valuable minerals are cerusite and smithsonite, and the gangue minerals are mainly quartz and calcite, followed by mica, gypsum, dolomite and chlorite. The phase analysis results of Pb and Zn are shown in Table 1, and the results show that the oxidation rates of Pb and Zn are 82.88% and 84.79%, respectively. The separation technology for the raw ore is determined as Pb flotation and then Zn flotation. Since the main aim of this study is to investigate the selective flotation of smithsonite, calcite and quartz, so the experimental sample was the tailings produced by the Pb beneficiation process of sulphidization and then flotation with xanthates.
A alkyl diamine ether (GE-609) of industrial pure was used as the collector in this investigation, and its structural formula is CH3(CH2)nCH2O(CH2)3NH(CH2)3- NH2, n=6-16. Solutions of HCl and NaOH were used to adjust pH value. Analytical grade sodium sulfide was used as an activator, and analytical grade sodium silicate and sodium hexametaphosphate were used as depressants. Distilled water was used for all experiments except the real ore flotation tests.
Table 1 Phase analysis results of Pb and Zn in raw ore (mass fraction, %)
2.2 Flotation experiment
The micro-flotation experiments were carried out in a 40 mL flotation cell at a constant stirring rate of 1600 r/min. For each test, 2 g of samples were separately conditioned with the corresponding reagents at a desired pH value for 3 min, and then floated for 4 min. At last, the concentrate and tailing were filtered, dried and weighed.
Flotation tests on the real ore sample were conducted in a series of 0.5-1.5 L flotation machine. The pH regulator, depressant and collector were successively added to perform the flotation. At last, the concentrate and tailing were separately dried, weighed and sampled to analyze the content of Zn to calculate the yield and recovery.
2.3 Zeta potential measurement
Zeta potentials of samples (-5 μm) were measured using a JS94H micro-electrophoresis meter (Shanghai, China). All measurements were conducted in a 1.0×10-3 mol/L KCl background electrolyte solution. The average value of three independent measurements was reported as the final result. The system error is less than 5%.
2.4 Infrared spectroscopy
The diffuse reflectance infrared spectra were obtained using a Nicolet FTIR-740 spectrometer (USA) to study the adsorption mechanism of GE-609 on smithsonite surface. Samples of -2 μm fraction were used and prepared according to the conditions of micro-flotation tests.
3 Results and discussion
3.1 Micro-flotation studies
Flotation performance of smithsonite, quartz and calcite as a function of pH using 25 mg/L GE-609 as the collector is shown in Fig. 1. It can be seen that a rise in pH from 6 to 10 improves the floatability of smithsonite with a maximum recovery of 85%. The recovery of quartz is approximately 100% in the pH range of 6-11, while it shows a sharp decrease when pH value is above 11. The floatability of calcite increases with the increase of pH value, and the flotation recovery is over 95% at pH 12.
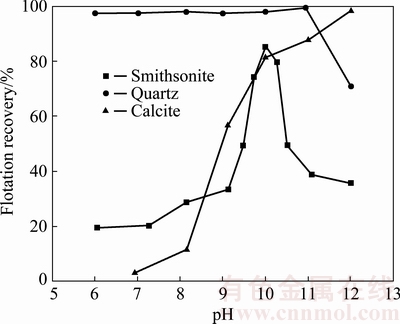
Fig. 1 Floatability of smithsonite, quartz and calcite as function of pH in the presence of 25 mg/L GE-609
The effects of GE-609 concentration on the floatability of smithsonite, quartz and calcite at pH 10 are shown in Fig. 2. It is observed that both smithsonite and quartz exhibit better floatability than calcite at low GE-609 concentrations (<40 mg/L), with the increase of GE-609 concentration from 10 to 100 mg/L, the recovery increases from 75% to 93% for smithsonite, and from 15% to nearly 100% for calcite. While for quartz, the recovery is decreased to 85% when GE-609 concentration increases to 50 mg/L after which the recovery increases. The results clearly show that GE-609 has good collecting performance to the three minerals, and it is necessary to use a depressant for both calcite and quartz in order to achieve the separation.
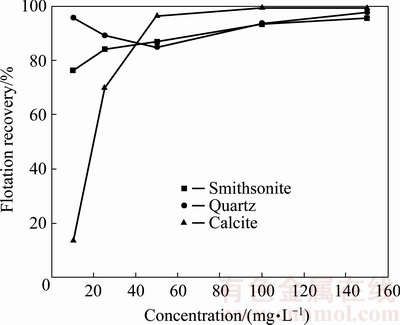
Fig. 2 Floatability of smithsonite, quartz and calcite as function of GE-609 concentration at pH 10
Figure 3 shows the effect of sodium sulfide on the floatability of smithsonite, quartz and calcite at natural pH (7.0-7.5) and 10 mg/L GE-609. When the concentration of sodium sulfide is less than 300 mg/L, the recovery of smithsonite increases with increasing the concentration. After that, the flotation recovery of smithsonite has a little decrease, and the maximum recovery is about 96%. This confirms that over- sulphidization of the pulp decreases flotation recovery due to the high content of HS- ion [6,10]. The calcite recovery increases with increasing sodium sulfide concentration, and it is about 90% when the concentration is above 500 mg/L. However, quartz shows a different flotation behavior, its recovery decreases as sodium sulfide concentration rises, and it is nearly 0 at 1000 mg/L sodium sulfide. Therefore, it is clearly show that the presence of sodium sulfide improves the flotation of smithsonite and calcite while it has depressing effect on quartz.
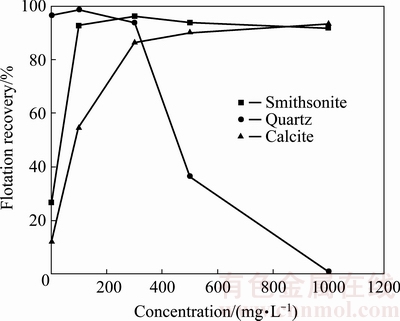
Fig. 3 Flotability of smithsonite, quartz and calcite as function of sodium sulfide concentration in the presence of 10 mg/L GE-609 at natural pH (7.0-7.5)
Using 10 mg/L GE-609 and 1000 mg/L sodium sulfide, the effects of sodium silicate and sodium hexametaphosphate concentration on the recovery of smithsonite and calcite were investigated, and the results are shown in Fig. 4. It can be seen that the recovery of calcite decreases from 90% to 10% at the sodium silicate concentration of 200 mg/L, where the recovery of smithsonite is still 95%. For sodium hexametaphosphate as the depressant, the recovery of calcite sharply decreases while the recovery of smithsonite has no obvious change, when the concentration is 3 mg/L, the recoveries of smithsonite and calcite are about 93% and 10%, respectively. According to these results, the flotation separation of smithsonite from calcite is possible to be achieved using sodium silicate or sodium hexametaphosphate as the depressant at an appropriate concentration.
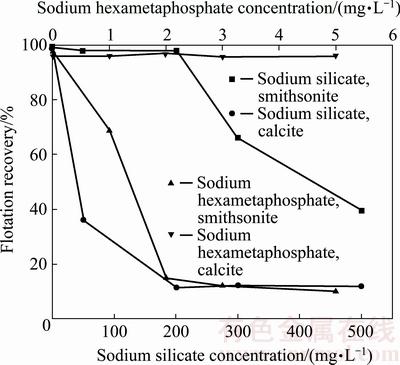
Fig. 4 Effect of depressant on flotation recovery of smithsonite and calcite
3.2 Beneficiation of smithsonite ore
In order to increase the zinc recovery, the beneficiation of smithsonite without desliming was carried out with the process of sulphidization-flotation with amines.
Based on the micro-flotation results and condition experiments, the reagents used in roughing operation are as follows: 3000 g/t Na2CO3, 400 g/t sodium hexameta- phosphate, 800 g/t sodium silicate, 8000 g/t sodium sulfide, and 400 g/t GE-609. By using the open-circuit flotation flowsheet of one-stage roughing, two-stage scavenging and three-stage cleaning, a zinc rough concentrate with 29.66% Zn was obtained. At last, a zinc concentrate containing 23.51% Zn with the recovery of 71.02% was obtained by the closed-circuit test in the laboratory [22].
3.3 Adsorption of GE-609 on smithsonite surface
3.3.1 Zeta potential characterization of smithsonite flotation solution
Smithsonite is a semi-soluble salt mineral, after adding it in water, the non-stoichiometric dissolution occurs, resulting in the hydrolysis of the ions released to solution. The dissolution species of smithsonite is complex. As the divalent transition element, Zn2+ inhabits multiple coordination environments owing to the ionic radius which is intermediate between the so-called radius ratio predictions for tetrahedral and octahedral coordination and the tendency to form more covalent bonds [23].
According to the distribution diagram plotted by HU et al [24], the species Zn2+ dominated until pH 8.1. The species Zn(OH)2(aq) dominated in the pH range of 8.1-11.5, while the species Zn(OH)3- and 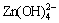 dominated above pH 11.5. STUMM and MORGAN [25] described that smithsonite was a thermo-dynamically stable solid below pH 7.4 but partially hydroxylated to hydrozincite in the pH range of 7.4-12.4.
dominated above pH 11.5. STUMM and MORGAN [25] described that smithsonite was a thermo-dynamically stable solid below pH 7.4 but partially hydroxylated to hydrozincite in the pH range of 7.4-12.4.
The zeta potential of smithsonite samples without treatment was measured, and the results (Fig. 5) show that the isoelectric point (IEP) of smithsonite is found to be pH 7.3 in water, which agrees with those reported in the pH range of 7-8 [5,26]. The surface of smithsonite is positively charged at pH<7.3, and negatively charged at higher pH.
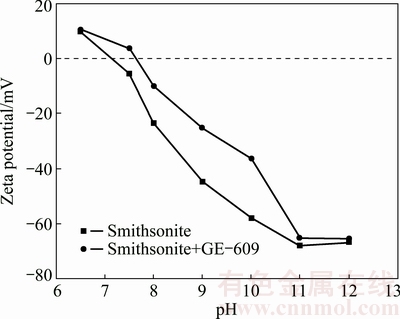
Fig. 5 Zeta potential of smithsonite as function of pH in the absence and presence of GE-609
In the presence of GE-609, a series of processes occur, including the dissolution of GE-609, the adsorption of amine ions on smithsonite surface, chemical and/or electrochemical reactions, and the possible dissolution of some surface species. The zeta potential of smithsonite after GE-609 treatment shows that adding GE-609 makes the zeta potential more positive and the value of isoelectric point increases. The pHIEP of smithsonite rises from 7.3 to about 7.7 because of the adsorption of GE-609.
As shown in Fig. 6, the species 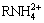 dominates when pH<10.98, whereas the species RNH2(s) and RNH2(aq) dominate when pH>10.98 [27]. Therefore, we can conclude that the more positive charge of smithsonite below pH 7.3 maybe be resulted from the reaction between
dominates when pH<10.98, whereas the species RNH2(s) and RNH2(aq) dominate when pH>10.98 [27]. Therefore, we can conclude that the more positive charge of smithsonite below pH 7.3 maybe be resulted from the reaction between 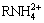 and
and 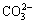 ions to form amine salts. In the pH range of 7.3-11, the smithsonite surface is negatively charged, the zeta potentials move to positive direction maybe due to the electrostatic adsorption of
ions to form amine salts. In the pH range of 7.3-11, the smithsonite surface is negatively charged, the zeta potentials move to positive direction maybe due to the electrostatic adsorption of 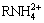 on smithsonite surface; whereas the zeta potentials almost keep unchanged above pH 11, possibly causing by the adsorption of RNH2(s) and RNH2(aq) on the smithsonite surface in the form of Zn-amine complexes [28].
on smithsonite surface; whereas the zeta potentials almost keep unchanged above pH 11, possibly causing by the adsorption of RNH2(s) and RNH2(aq) on the smithsonite surface in the form of Zn-amine complexes [28].
3.3.2 Infrared spectroscopy
Figure 7 shows the spectra of GE-609 adsorbed on smithsonite surface in the presence and absence of sodium sulfide. As can be seen, the bands at 3381 and 3329 cm-1 in the spectrum of GE-609 (a) are due to the stretching vibration of —NH2, 1622 and 1570 cm-1 are due to the bending vibration of —NH2 and +NH3. The bands at 2924 and 2854 cm-1 are assigned to —CH3 and —CH2 stretching. The adsorption band at 719 cm-1 is due to the in-plane rocking vibration of —(CH2)n— (n≥4). Besides, the characteristic band at 1115 cm-1 is due to the stretching vibration of C—O—C group [27]. The smithsonite spectrum (b) displays several bands, the bands at 870 and 743 cm-1 correspond to the bending vibrations of 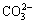 , 1444 cm-1 is due to the asymmetric stretching vibration of
, 1444 cm-1 is due to the asymmetric stretching vibration of 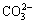 . The wide band at 3325 cm-1 is due to the stretching vibration of—OH [12]. After being treated with sodium sulfide (c), the C=O bands at 1444 cm-1 partially disappear to form partially ZnS on the smithsonite surface. In the spectrum (d), there are several new bands after GE-609 treatment. Two new bands are observed at 2854 and 2923 cm-1, assigned to —CH3 and —CH2 stretching. Two new bands at 3433 and 3334 cm-1 are due to the stretching vibration of —NH2. And a new band at 1180 cm-1 is due to the stretching vibration of C—O—C group. Mean- while, the characteristic smithsonite peak of 1444 cm-1 assigned to
. The wide band at 3325 cm-1 is due to the stretching vibration of—OH [12]. After being treated with sodium sulfide (c), the C=O bands at 1444 cm-1 partially disappear to form partially ZnS on the smithsonite surface. In the spectrum (d), there are several new bands after GE-609 treatment. Two new bands are observed at 2854 and 2923 cm-1, assigned to —CH3 and —CH2 stretching. Two new bands at 3433 and 3334 cm-1 are due to the stretching vibration of —NH2. And a new band at 1180 cm-1 is due to the stretching vibration of C—O—C group. Mean- while, the characteristic smithsonite peak of 1444 cm-1 assigned to 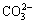 vibration absorption has shifted to 1435 cm-1. In the spectrum (e), the presence of sodium sulfide makes the intensity of alkyl chain bands and —NH2 bands increase, which indicates the increased adsorption of GE-609 on smithsonite surface.
vibration absorption has shifted to 1435 cm-1. In the spectrum (e), the presence of sodium sulfide makes the intensity of alkyl chain bands and —NH2 bands increase, which indicates the increased adsorption of GE-609 on smithsonite surface.
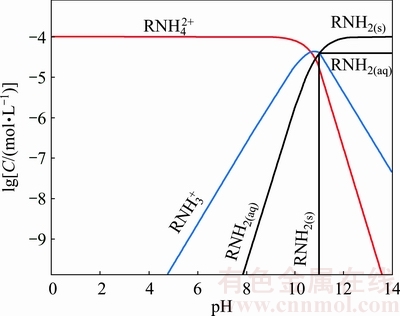
Fig. 6 Distribution of various species in GE-609 solution as function of pH ([GE-609]=1×10-4 mol/L)
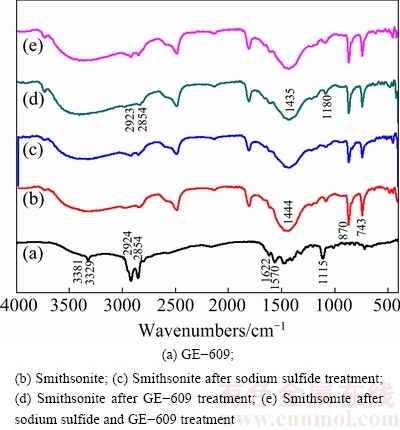
Fig. 7 Infrared spectra of GE-609 and smithsonite
4 Conclusions
1) The alkyl diamine ether GE-609 exhibits good collecting capability to smithsonite, calcite and quartz, and it is unavoidable to use a depressant for their separation.
2) Sodium sulfide can improve the flotation of smithsonite and calcite while has depressing effect on quartz, both sodium silicate and sodium hexameta- phosphate have selective inhibition to calcite at an appropriate concentration.
3) Zeta potential and FTIR analysis show that both electrostatic and chemical adsorption of GE-609 occur on smithsonite surface, and the presence of sodium sulfide enhances the adsorption of GE-609.
References
[1] KASHANI A H N, RASHCHI F. Separation of oxidized zinc minerals from tailings: Influence of flotation reagents [J]. Minerals Engineering, 2008, 21(12-14): 967-972.
[2]  G, BULUT G, GüL A, PEREK K T, ARSLAN F. Flotation of
G, BULUT G, GüL A, PEREK K T, ARSLAN F. Flotation of  oxide lead-zinc ores [J]. Minerals Engineering, 2005, 18(2): 279-282.
oxide lead-zinc ores [J]. Minerals Engineering, 2005, 18(2): 279-282.
[3] HOSSEINI S H, FORSSBERG E. Physicochemical studies of smithsonite flotation using mixed anionic/cationic collector [J]. Minerals Engineering, 2007, 20(6): 621-624.
[4] PEREIRA C A, PERES A E C. Reagents in calamine zinc ores flotation [J]. Mineral Engineering, 2004,18(2): 275-277.
[5] SHI Qing, FENG Qi-ming, ZHANG Guo-fan, DENG Hong. Electrokinetic properties of smithsonite and its floatability with anionic collector [J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2012, 410: 178-183.
[6] EJTEMAEIM, IRANNAJAD M,GHARABAGHI M. Role of dissolved mineral species in selective flotation of smithsonite from quartz using oleate as collector [J]. International Journal of Mineral Processing, 2012, 114-117: 40-47.
[7] MARABINI A M, CIRIACHI M, PIESCIA P, BARBARO M. Chelating reagents for flotation [J]. Minerals Engineering, 2007, 20(10): 1014-1025.
[8] HOSSEINI S H, FORSSBERG E. Adsorption studies of smithsonite flotation using dodecylamine and oleic acid [J]. Minerals & Metallurgical Processing, 2006,23 (2): 87-96.
[9] KIERSZNICKI T, MAJEWSKI J, MZYK J. 5-Alkylsalicylaldoximes as collectors in flotation of sphalerite, smithsonite and dolomite in a Hallimond tube [J]. International Journal of Mineral Processing, 1981, 7(4): 311-318.
[10] KEQING F A, MILLER J D, JIANG Tao, LI Guang-hui. Sulphidization flotation for recovery of lead and zinc from oxide-sulfide ores [J]. Transactions of Nonferrous Metals Society of China, 2007, 15 (5): 1138-1147.
[11] MEHDILO A, ZAREI H, IRANNAJAD M, ARJMANDFAR H. Flotation of zinc oxide ores by cationic and mixed collectors [J]. Minerals Engineering, 2012, 36-38(10): 331-334.
[12] JANUSZ W, SZYMULA M, SZCZYPA J. Flotation of synthetic zinc carbonate using potassium ethyl xanthate [J]. International Journal of Mineral Processing, 1983, 48(2): 40-44.
[13] HOSSEINI S H, FORSSBERG E. Smithsonite flotation using potassium amyl xanthate and hexylmercaptan [J].Mineral Processing and Extractive Metallurgy IMM Transactions (section C), 2013, 115(2): 107-112.
[14] EJTEMAEIM, IRANNAJAD M, GHARABAGHI M. Influence of important factors on flotation of zinc oxide mineral using cationic, anionic and mixed (cationic/anionic) collectors [J]. Minerals Engineering, 2011, 24(13): 1402-1408.
[15] APLAN F F, de BRUYN P L. Adsorption of hexyl mercaptan on gold [J]. Transaction AIME, 1963, 229: 235-242.
[16] SALUM M J G, de ARAUJO A C, PERES A E C. The role of sodium sulphide in amine flotation of silicate zinc minerals [J]. Minerals Engineering, 1992, 5(3-5): 411-419.
[17] MCKENNA W J, LESSELS V, PETERSSON E C. Froth flotation of oxidized zinc ores: US patent, 2482859 [P].1949.
[18] ABRAMOV A A. Use of cationic agents for oxide lead-zinc minerals [J]. Chemical Abstracts, 1961, 55: 26910f.
[19] AL-THYABAT S. Evaluation of mechanical flotation of non-slimed Jordanian siliceous phosphate [J]. Arabian Journal for Science and Engineering, 2012, 37: 877-887.
[20] GE Ying-yong, YU Yong-fu, CHEN Da, ZHANG Ming. Flotation performance of low-temperature resistant cationic collector GE-609 using in separating SiO2 [J]. Journal of Wuhan University of Technology, 2005, 27(8): 17-19. (in Chinese)
[21] GE Ying-yong, LIU Jing, WANG Kai-jin, MEI Guang-jun. Investigation on reverse flotation of Lanxian hematite ore by GE-609 cationic collector [J]. Metal Mine, 2006(8): 183-185. (in Chinese)
[22] LI Lai-shun, LIU San-jun, ZHU Hai-ling, ZHANG Ting, QIN Wen-qing. Experimental research on beneficiation of lead-zinc oxide ore from Yunnan [J]. Mining and Metallurgical Engineering of China, 2013, 33(3): 69-73. (in Chinese)
[23] WELLS A F. Structural inorganic chemistry [M]. 5th ed. Oxford: Clarendon Press, 1984.
[24] HU Yue-hua, XU Jin, LUO Chao-qi, YUAN Cheng. Solution chemistry studies on dodecylamine flotation of smithsonite/calcite [J]. Journal of Central South University of Technology, 1995, 26: 589-594.
[25] STUMM W, MORGAN J J. Aquatic chemistry [M]. New York: Wiley Inter Science, 1970.
[26] WU Dan-dan,WEN Shu-ming,DENGJiu-shuai.LIU Jian, MAO Ying-bo. Study on the sulfidation behavior of smithsonite [J]. Applied Surface Science, 2015, 329: 315-320.
[27] GE Ying-yong. Synthesis of a new collector alkyl polyamine ether (GE-609) and researcher of its flotation behavior [D]. Wuhan: Wuhan University of Technology, 2010. (in Chinese)
[28] HU Yue-hua, WANG Dian-zuo. Flotation theory and practice of lead-zinc oxide ores—A review of the lead-zinc oxide minerals flotation in other countries [J]. Non-Ferrous Mining and Metallurgy of China, 1986, (2): 14-22. (in Chinese).
捕收剂烷基二醚胺对菱锌矿、石英和方解石选择性浮选的影响
朱海玲1,2,覃文庆2,陈 臣2,柴立元1,李来顺2,刘三军2,张 婷2
1. 中南大学 冶金与环境学院,长沙 410083;
2. 中南大学 资源加工与生物工程学院,长沙 410083
摘 要:通过单矿物浮选试验和实际矿石浮选试验考察了烷基二醚胺(GE-609)做捕收剂时,菱锌矿、石英和方解石的浮选分离。结果表明,GE-609对3种矿物均有良好的捕收性能,浮选无选择性。硫化钠能增强菱锌矿和方解石的浮选但抑制石英。此外,水玻璃和六偏磷酸钠均对方解石表现出良好的选择性抑制作用。实际矿石浮选试验表明,最终闭路试验获得Zn品位为23.51%、回收率为71.02%的锌精矿。通过动电位测试和红外光谱分析考察了GE-609在菱锌矿表面的吸附,结果表明,GE-609在菱锌矿表面的吸附包括静电吸附和化学吸附,且硫化钠的存在增强了BGE-609在菱锌矿表面的吸附。
关键词:菱锌矿;石英;方解石;烷基二醚胺;硫化钠;硫化浮选
(Edited by Xiang-qun LI)
Foundation item: Project (2016RS2016) supported by Innovation Team of Interface Chemistry of Efficient and Clean Utilization of Complex Mineral Resources, China
Corresponding author: Wen-qing QIN; Tel: +86-731-88876843; E-mail: qinwenqing369@126.com
DOI: 10.1016/S1003-6326(18)64649-7
Abstract: The flotation separation of smithsonite from calcite and quartz using a alkyl diamine ether (GE-609) as the collector was investigated through micro-flotation experiments and the real ore flotation experiments. The results show that GE-609 exhibits good collecting capability to three minerals without selectivity. The presence of sodium sulfide enhances the flotation of smithsonite and calcite while inhibits quartz. Moreover, both sodium silicate and sodium hexametaphosphate exhibit good selective inhibition to calcite. The real ore test results show that a zinc concentrate containing 23.51% Zn with the recovery of 71.02% is obtained in the closed-circuit test. To understand the adsorption of GE-609 on smithsonite surface, zeta potential measurement and FTIR analysis were carried out, and the results indicate that the collector GE-609 can adsorb on smithsonite surface through both electrostatic adsorption and chemical adsorption, and the presence of sodium sulfide enhances the adsorption of GE-609.


