
Thermodynamic analysis on synthesis of fibrous Ni-Co alloys precursor and Ni/Co ratio control
ZHAN Jing1, 2, HE Yue-hui1, ZHOU Di-fei2, ZHANG Chuan-fu2
1. State Key Laboratory for Powder Metallurgy, Central South University, Changsha 410083, China;
2. School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China
Received 14 October 2010; accepted 3 April 2011
Abstract:
According to the principles of simultaneous equilibrium and mass equilibrium, a series of thermodynamic equilibrium equations in Ni(II)-Co(II)-C2O42--NH3-NH4+-H2O system at ambient temperature were deduced. The diagrams of logarithm ion concentrations versus pH values at different solution compositions were drawn. The results show that Ni2+ and Co2+ can completely precipitate at pH less than 5.0 and the predefined Ni/Co ratios can be well kept in the precursor. The precursor morphology is granular aggregation. However, rod aggregation precursor is obtained in the pH range of 5.0-8.0, and fibre-shape precursor is got at pH value higher than 8.0. The Ni/Co ratios in the above two precursors are not reproduced as that in the feed due to the formation of multi-coordinated Ni(NH3)n2+ and Co(NH3)n2+ (n=1-6). Modification of precipitation medium is favorable for the precursors to keep the predefined Ni/Co ratios of the feed in the pH range of 2.0-8.6. Meanwhile, the precursors with fibrous morphology can be obtained.
Key words:
thermodynamic analysis; nickel-cobalt alloy; nickel-cobalt oxalate precursor; predefined Ni/Co ratio; fibre-shape;
1 Introduction
As important magnetic materials, Ni-Co alloys have potential application in high-density magnetic recording medias, ferrofluids technology, magnetic resonance imaging, high-temperature catalysts, microwave absorber and electromagnetic shielding material[1]. Recently, the preparation methods and applications of one-dimensional (1D) Ni-Co alloy materials have been paid to more attention due to their unique properties of low dimensionality[2]. The preparation methods include template[3], liquid-phase reduction[4-5], electro- deposition[6], microemulsion[7], sol-gel-thermal reduction[8], magnetic-field-induced assembly[9], and hydrothermal method[10]. However, the above synthesis methods would result in difficulties in application of magnetic assemblies in nanodevices, owing to the presence of the surfactants or the requirement of external magnetic fields. Up to date, few 1D assembly of Ni-Co alloys was independent of any surfactants or external forces.
In previous research[11-13], fibrous nickel, copper and NiO powders have been prepared in the system of Me(II)-C2O42--NH3-NH4+-H2O by complex precipitation-thermal decomposition (Me represents nickel, cobalt or copper). This reaction system is probably suitable to synthesize fibrous Ni-Co alloys. ZHANG et al[14] prepared fibrous Ni-Co alloy precursors by oxalate coprecipitation method in the system of Ni(II)-Co(II)-C2O42--NH3-NH4+-H2O. However, compositional homogeneity and maintenance of predefined Ni/Co ratios in the prepared Ni-Co alloy are not solved because of different thermodynamic properties of the precipitation reaction for each metal ion and preferential precipitation of nickel oxalate or cobalt oxalate. Recently, PUJOL et al[15] conducted the research of nanostructured cobalt oxalate precipitation by using cryogenic electron microscopy technique to characterize intermediate precipitation products. They found a strong correlation between the particle evolution and the solution chemistry. The particle evolution was related to the ionic concentration in solution and pH value. ZHAN et al[16] reported the shape-controlled synthesis of the fibrous Ni-Co alloys precursor with desired Ni/Co ratios by improving reaction conditions. The final precipitate properties of Ni-Co alloys precursor including composition, particle size and morphology were obviously affected by the complex formation in the reaction system, their sensitivity to pH and the simultaneous presence of several species. Therefore, this work aims to further identify process by the thermodynamic calculation of Ni(II)-Co(II)-C2O42-- NH3-NH4+-H2O system and to understand correlation between the maintenance of the predefined Ni/Co ratio and fibrous morphology. Based on the thermodynamic calculation and the improved experiments, the fibrous Ni-Co alloys precursor with predefined Ni/Co are prepared.
2 Thermodynamic calculation of Ni(II)- Co(II) -C2O42--NH3-NH4+-H2O system
2.1 Principle of calculation method
In the system of Me(II)-NH3-NH4+-C2O42--H2O, there exist not only precipitation reactions, but also diversified coordination reaction. Regarding the reaction between Me2+ and C2O42-, it can be expressed as follows:
Me2++C2O42-=MeC2O4(s)
Ksp(MeC2O4(s))=[Me2+][C2O42-] (1)
For the reaction between Me2+ and OH-, there are following formula:
Me2++2OH-=Me (OH)2(s)
Ksp(Me(OH)2(s))=[Me2+][OH-]2 (2)
Therefore, the actual concentration of free Me2+ in the solution is deduced by using following equation:
[Me2+]=min{Ksp(MeC2O4(s))/[C2O42-],
Ksp(Me(OH)2(s))/[OH-]2} (3)
2.2 Thermodynamic data and equilibrium equations
In the system of Ni(II)-Co(II)-C2O42--NH3- NH4+-H2O, the main chemical reactions include the coordination reaction of Ni2+ and Co2+ with ammonia and C2O42-, hydrolysis of Ni2+ and Co2+, compounding of Ni2+ and Co2+ with C2O42-, and dissociation of weak acids and alkaline. The relevant reactions are listed in Table 1. For a certain precipitation process, the variables, such as temperature and pressure, are usually constant, so the diagrams of lg[Me2+]T versus pH values at different solution compositions would be drawn for further discussion.
2.3 Mathematical model
Based on the principles of simultaneous equilibrium and mass equilibrium, the mathematic models of lg[Me2+]T vs pH value at different solution compositions are deduced. [Ni2+], [Co2+], [Ni2+]T, [Co2+]T, [NH3]T, and [C2O42-]T represent the free concentrations of Ni2+ and Co2+, the total concentrations of Ni2+, Co2+, ammonia and C2O42-, respectively.
Table1 Equilibrium equations and equilibrium constants in Ni(II)-Co(II)-C2O42--NH3-NH4+-H2O system (T=298K)[17]
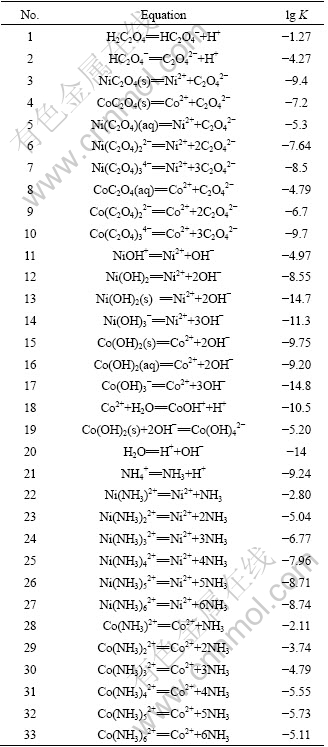
The total concentrations of coordinations of Ni2+ and Co2+ with ammonia in solution are expressed as [NiNH3]T and [CoNH3]T, respectively. Both expressions are shown as follows:
[NiNH3]T=[Ni(NH3)2+]+[Ni(NH3)22+]+[Ni(NH3)32+]+
[Ni (NH3)42+]+[Ni(NH3)52+]+[Ni(NH3)62+]
=[Ni2+](102.80[NH3]+105.04[NH3]2+106.77[NH3]3+
107.96[NH3]4+108.71[NH3]5+108.74[NH3]6) (4)
[CoNH3]T=[Co(NH3)2+]+[Co(NH3)22+]+[Co(NH3)32+]+
[Co (NH3)42+] +[Co(NH3)52+]+[Co(NH3)62+]
=[Co2+](102.11[NH3]+103.74[NH3]2+104.79[NH3]3+
105.55[NH3]4+105.73[NH3]5+105.11[NH3]6) (5)
The total concentrations of coordination of Ni2+ and Co2+ with OH- are expressed as [NiOH] T and [CoOH] T in solution, respectively. The equations are listed as follows:
[NiOH]T=[Ni(OH)+]+[Ni(OH)2(aq)]+[Ni(OH)3-]
=[Ni2+](10pH-9.03+102pH-19.45+103pH-30.67) (6)
[CoOH]T=[Co(OH)+]+[Co(OH)2(aq)]+
[Co(OH)3-]+[Co(OH)42-]
=[Ni2+](10pH-9.03+102pH-19.45+103pH-30.67) (6)
[CoOH]T=[Co(OH)+]+[Co(OH)2(aq)]+
= [Co2+](10pH-9.75+102pH-18.4+
103pH-31.5+102pH-33.10) (7)
The total concentrations of coordination of Ni2+ and Co2+ with C2O42- are expressed as [NiC2O4]T and [CoC2O4]T, respectively. The following equations are obtained:
[NiC2O4]T=[Ni(C2O4)(aq)]+[Ni(C2O4)22-]+[Ni(C2O4)34-]
=[Ni2+](105.3[C2O42-]+107.64[C2O42-]2+108.5[C2O42-]3)(8)
[CoC2O4]T=[Co(C2O4)(aq)]+[Co(C2O4)22-]+[Co(C2O4)34-]
=[Co2+](104.79[C2O42-]+106.7[C2O42-]2+109.7[C2O42-]3)(9)
Thus, the total concentrations of C2O42-, NH3, Ni2+ and Co2+, namely [C2O42-]T, [NH3]T, [Ni2+]T and [Co2+]T can be obtained with following equations:
[C2O42-]T=[C2O42-]+[HC2O4-]+[H2C2O4]+
[NiC2O4]T+[CoC2O4]T
=[C2O42-]{(1+105.52-2pH+104.27-pH)+
[Ni2+](105.3+2×107.64[C2O42-]+
3×108.5[C2O42-]2)+[Co2+](104.79+
2×106.7[C2O42-]+3×109.7[C2O42-]2)} (10)
[NH3]T=[NH3]+[NH4+]+[NiNH3]T+[CoNH3]T
=[NH3](1+109.24-pH)+[Ni2+](102.80[NH3]+
2×105.04[NH3]2+3×106.77[NH3]3+4×107.96[NH3]4+
5×108.71[NH3]5+6×108.74[NH3]6)+[Co2+](102.11[NH3]+
2×103.74[NH3]2+3×104.79[NH3]3+4×105.55[NH3]4+
5×105.73[NH3]5+6×105.11[NH3]6) (11)
[Ni2+]T=[Ni2+]+[NiOH]T+[NiC2O4]T+[NiNH3]T
=[Ni2+]{1+10pH-9.03+102pH-19.45+103pH-30.67+
105.3[C2O42-]+107.64[C2O42-]2+108.5[C2O42-]3+
102.80[NH3]+105.04[NH3]2+106.77[NH3]3+
107.96[NH3]4+108.71[NH3]5+108.74[NH3]6} (12)
[Co]T=[Co2+]+[CoOH]T+[CoC2O4]T+[CoNH3]T
=[Co2+]{1+10pH-9.75+102pH-18.4+103pH-31.5+102pH-33.10+
104.79[C2O42-]+106.7[C2O42-]2+109.7[C2O42-]3+
102.11[NH3]+103.74[NH3]2+104.79[NH3]3+105.55[NH3]4+
105.73[NH3]5+105.11[NH3]6} (13)
According to the principle of calculation, the actual concentration of free Ni2+ and Co2+ in the solution can be expressed as follows:
[Co2+]=min{Ksp(CoC2O4(s))/[C2O42-],
Ksp(Co(OH)2(s))/[OH-]2} (14)
[Ni2+]=min{Ksp(NiC2O4)(s)/[C2O42-],
Ksp(Ni(OH)2(s))/[OH-]2} (15)
3 Calculated results and discussion
The relationships among the nine variables of [Co]T , [Co2+], [Ni2+]T, [Ni2+], [NH3]T, [NH3], [C2O42-]T, [C2O42-], and pH are confined by Eqs.(4)-(15). For the given values of [C2O42-]T and [NH3]T at a certain pH value, other variables can be obtained from the above equations by computation. The calculated results are plotted as shown in Figs.1-3.
Figure 1 shows the curves of lg[Ni2+]T and lg[Co2+]T vs pH value in Ni(II)-Co(II)-C2O42--NH3-NH4+-H2O system (T=298 K) at different total ammonia concen- trations with a fixed [C2O42-]T of 0.6 mol/L. It can be observed when pH value is less than 4.0, the total concentrations of nickel and cobalt ions, [Ni2+]T and [Co2+]T decrease with increasing pH value. In the pH value range from 4.0 to 5.5, [Ni2+]T and [Co2+]T almost reach the minimum values. However, [Ni2+]T and [Co2+]T increase sharply with increasing pH values in the range of 5.5-9.0 for [Ni2+]T and 5.5-9.5 for [Co2+] due to the coordination of nickel and cobalt ions with ammonia. The higher the ammonia concentration is, the higher [Ni2+]T and [Co2+]T are. [Ni2+]T and [Co2+]T almost keep constant at the maximum value due to the formation of Ni(NH3)2+6 and Co(NH3)2+6 in the pH range of 9.5-11.0 for nickel ions and 9.0-10.0 for cobalt ions. High alkaline condition, such as pH>11.0 for nickel ions and pH>10.0 for cobalt ions, causes declined concentrations of [Ni2+]T and [Co2+]T again because nickel ammonia or cobalt ammonia complex will dissociate to nickel ions and cobalt ions under high pH conditions. The dissociated metal ions precipitate with OH-, leading to the decrease of total concentrations of nickel and cobalt ions in the solution.
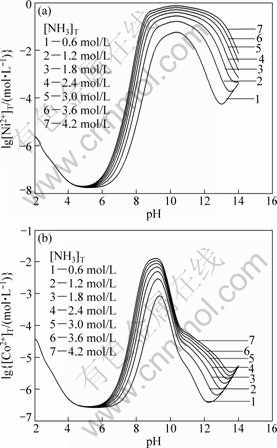
Fig.1 Curves of lg[Ni2+]T and lg[Co2+]T vs pH in Ni(II)- Co(II)-NH3-NH4+-C2O42--H2O system at [C2O4]2-T=0.6 mol/L and different [NH3]T (T=298 K)
In addition, [Ni2+]T and [Co2+]T increase with increasing ammonia concentration because of the presence of complex reaction between Ni2+, Co2+ and ammonia. When [NH3]T increases, [Ni2+]T and [Co2+]T almost maintain stable at pH<5.5 for Ni2+ and pH<6.0 for Co2+. However, in the pH range of 5.5-11.0 for Ni2+ and 6.0-10.0 for Co2+, [Ni2+]T and [Co2+]T gradually increase with increasing [NH3]T due to the strengthened complex reaction of Ni2+ and Co2+ with ammonia. The higher the concentration of ammonia is, the higher [Ni2+]T and [Co2+]T in the solution are. The stability constant of soluble complexes between Ni2+and ammonia is larger than that between Co2+ and ammonia. The solubility of NiC2O4 (0.36 g/L at 291 K) is larger than that of CoC2O4 (0.021 g/L), so the coprecipitation production cannot keep the predefined Ni/Co ratios of the feed.
Figure 2 shows the curves of lg[Ni2+]T and lg[Co2+]T vs pH value in the studied system at different [C2O42-]T (T=298 K) and [NH3]T being 2.0 mol/L. The results show the total concentrations of Ni2+ and Co2+ in the solution decrease with increasing [C2O42-]T because coprecipitation between Ni2+ and Co2+ with C2O42- is susceptible to achieve complete precipitation under excess chemical stoichiometry of oxalic acid at pH<5.0. The effects of the total concentrations of C2O42- on the precipitation rate of metal ions are more obvious at pH>8.0. Therefore, the total C2O42- concentration is crucial during precipitation process. In order to reduce the effect of the total C2O42- concentration on the precipitation rate of metal ions, C2O42- total concentration should be controlled at 1.0-1.2 times of its stoichiometry.
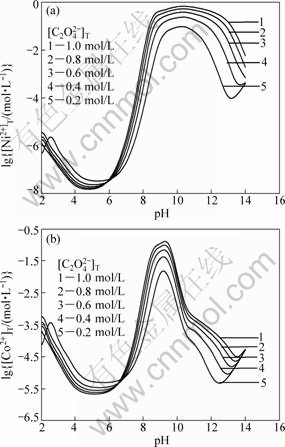
Fig.2 Curves of lg[Ni2+]T and lg[Co2+]T vs pH in Ni(II)- Co(II)-C2O42--NH3-H2O system at [NH3]T=2.0 mol/L and different [C2O42-]T (T=298 K)
Figure 3 shows the curves of lg c vs pH value for each nickel and cobalt species in solution system (T=298 K) with [C2O42-]T=1.0 mol/L and [NH3]T=3.0 mol/L. It can be found that the concentrations of free nickel or cobalt ions are the dominant species in the solution. Partially, Ni(NH3)n2+ and Co(NH3)n2+ (n=1, 2) are formed at pH<6.0 and pH<7.0, respectively. Moreover, the dominant species are Ni(NH3)n2+ and Co(NH3)n2+ (n=3-6) in the pH range of 6.0-10.5 for Ni2+ and 7.0-13.0 for Co2+, respectively, because most of nickel and cobalt ions coordinate with ammonia, resulting in the concentrations of free nickel and cobalt ions gradually decrease with increasing ammonia concentrations. The maximum concentrations of nickel ammonia and cobalt ammonia complex are obtained in the pH range from 10.5 to 13.0. The concentrations of nickel and cobalt hydroxide complex become the dominate ions at pH value higher than 13.0.
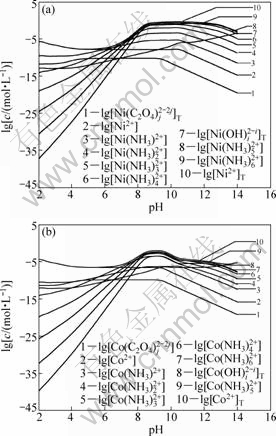
Fig.3 Curves of lg c vs pH for each nickel ion species and each cobalt ion species in Ni(II)-Co(II)-NH3-NH4+- C2O42--H2O system at [C2O4]2-T=1.0 mol/L and [NH3]T=3.0 mol/L (T=298 K)
As mentioned above, the coprecipitation is a complicated dynamical equilibrium process and different ion species compete against each other in Ni(II)-Co(II)- NH3-NH4+-C2O42--H2O system. The rate and order of precipitation for each metal ion are dependent on precipitation-dissolution equilibrium constant, initial concentration of precipitation agent, stability constant of soluble complex and dissociation equilibrium constant. When pH value is less than 5.0, the nickel and cobalt ions can be considered as complete coprecipitation and the predefined Ni/Co ratios in the feed can be well maintained in the coprecipitation product. When pH value is in the range of 5.0-9.0, there exist the coordination reaction between metal ions and ammonia, and the coprecipitation of Ni2+ and Co2+ with C2O42-. The coprecipitate cannot maintain the predefined Ni/Co ratios in the feed in the pH range of 5.0-9.0 and cobalt content in the precipitate is larger than the nickel content.
4 Results and discussion
4.1 Experimental process
NiCl2?6H2O, CoCl2?6H2O, NH3?H2O, C2H5OH, CH3COCH3, (NH4)2C2O4, H2C2O4 and surfactant PVP were used as starting materials. All of the reagents were analytical grade without further purification prior to use. Nickel chloride and cobalt chloride were mixed in a container according to different ratios of Ni2+ to Co2+. Then the intimate mixture was injected into water solvent or mixture-solvent medium containing oxalate or ammonium oxalate, organic solution and ammonia by a peristaltic pump under certain temperature. Meanwhile, ammonia solution was added to adjust and stabilize the pH value. After agitating and aging, the coprecipitate was washed with distilled water followed by surfactant solution, ethanol and acetone. Finally, the coprecipitates were filtered and dried in vacuum at 373-393 K for more than 12 h, and then the precursor particles were obtained.
The residual contents of Ni 2+ and Co2+ in the filtrate were analyzed by atomic absorption spectrometry (AAS). The morphology of the precursor was observed with an electron scanning microscope (SEM) (JSM-5600LV). The contents of Ni2+ and Co2 in the precursor were determined by chemical analysis and energy dispersive spectrometry (EDS) for SEM. The Ni/Co molar ratio (R) can be calculated by
R=(w(Ni)?m/A(Ni))/(w(Co)?m/A(Co)) (16)
where w(Ni) and w(Co) represent Ni2+ and Co2+ mass fraction in the sample, respectively, m represents the mass of the sample, A(Ni) and A(Co) represent Ni and Co relative atomic mass, respectively.
4.2 Relationship between precursor morphology and pH value in solution
Figure 4 shows the correlation between the morphology of the precipitated particles and their formation conditions based on the experimental and theoretical results. From Fig.4, it can be seen that the granular aggregation particles are obtained when pH is below 5.0, while the rod aggregation particles are present in pH range from 5.0 to 8.0; and the fibre-shape particles are obtained at pH value higher than 8.0. This can probably attribute to the increase of free ammonia amount combined with nickel-cobalt oxalate in solution. Theoretically, the concentrations of free ammonia and various nickel-cobalt ammonia complexes increase gradually with increasing pH in the solution when pH value varies from 0 to 12 (Fig.3). As a result, the process of particle nucleation and growth is changed and diverse morphologies of particles are formed at different pH values. Undoubtedly, the different solution composition leads to the formation of these three kinds of particles with different shapes. In order to obtain certain precursor particles, the pH value should be controlled in the corresponding range by using ammonia. In terms of this figure, the pH value range should be adjusted above 8.0.
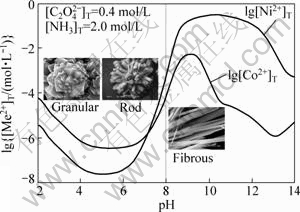
Fig.4 Correlation between morphology of coprecipitated particles and corresponding formation conditions in Ni(II)- Co(II)-NH3-NH4+-C2O42--H2O system
4.3 Effect of pH on maintenance of predefined ratio of Ni to Co in water solvent and mixed solvent
Figure 5 shows the influence of pH value on the ratio of Ni2+ to Co2+ in coprecipitation product in water solvent, under the conditions of predefined Ni2+/Co2+ molar ratio in the feed of 4:1, reaction time of 1 h, reaction temperature of 333 K, aging time of 3 h, and the total concentration of Ni2+ and Co2+ of 0.5 mol/L. From Fig.5, it can be concluded that the predefined Ni/Co molar ratios in the feed can be well maintained in the precursor at pH<5.0. When pH value is in the range of 5.0-9.0, the predefined Ni/Co molar ratio in the feed cannot be kept in the precursor, which indicates that the experimental result is in accordance with theoretically calculated values. Table 2 shows the EDS analysis of Ni2+ and Co2+ content in different selected areas of the coprecipitates with initial mass ratios of Ni2+to Co2+ of 84:16 and 67:33. The results show that not only the predefined Ni/Co ratio cannot be maintained but also the composition is heterogeneous in the coprecipitate product. From the above analysis, it is found that the pH value higher than 6.0 does not favor the complete precipitation of Ni2+, which results in difficulty to obtain a desired pH range to maintain predefined Ni/Co ratio with fibrous morphology for Ni-Co alloy precursor.
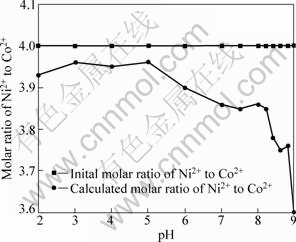
Fig.5 Influence of different pH values on molar ratio of Ni to Co in coprecipitate when water solvent is used as precipitating medium
In order to synthesize the fibrous Ni-Co alloy precursor particles with original Ni/Co ratios of the feed and compositional homogeneity, the solvent should be selected to ensure the presence of a wide pH range to maintain original Ni/Co ratio in the coprecipitation process. A modified coprecipitation process is presented based on the principle of physical chemistry, where the solvent is composed of water and organic solvent with surface tension lower than water (labeled as X). The X can mix with water at arbitrary ratio. In this new solvent (hereinafter defined as mixed solvent), the volume fraction of X is always higher than 25%.
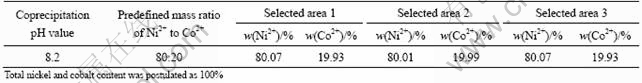
Figure 6 shows the influence of pH value on the precipitating rate of metal ions(calculated according to residual metal ions of filtrate)and the Ni/Co molar ratios in the precursor (calculated according to solid coprecipitate) in the modified coprecipitation under the above conditions, except for the mixed solvent of X and water (25%) instead of water solvent used as precipitating medium and different predefined Ni2+/Co2+ in the feed. It is seen from Fig.6(a) that the precipitating rate of Ni2+ and Co2+, especially Ni2+, obviously increased by changing precipitating medium. The reason could contribute to the solubility of individual cation oxalate and soluble complex of each cation in X lower than those in water medium, which results in the reduction of each cation residual concentration. From Fig.6(b), it is concluded that precipitation could maintain the original Ni/Co ratio in the feed excellently at 2.0≤pH<8.6. Table 3 shows EDS analysis of Ni 2+ and Co2+ content in different selected areas of the coprecipitates. The results show that the modified coprecipitation process can improve the maintenance of predefined Ni/Co ratios in the feed and compositional homogeneity of cation for the fibrous Ni-Co alloy precursor.
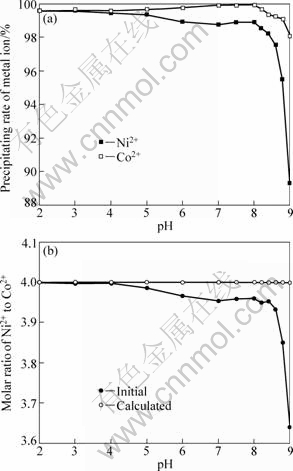
Fig.6 Influence of pH on precipitating rate of metal ions (a) and Ni/Co molar ratio of coprecipitate (b)
Table 2 Ni2+ and Co2+ content of as-prepared fibrous precursor with different predefined Ni/Co mass ratios by EDX
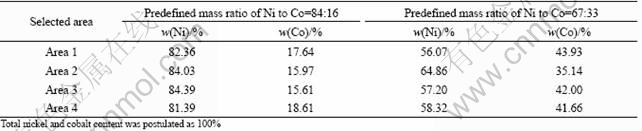
Table 3 Ni2+ and Co2+ content of as-prepared fibrous precursor with predefined mass ratio of Ni to Co=80:20
5 Conclusions
1) Based on the principles of simultaneous equilibrium and mass equilibrium, a serials of thermodynamic equilibrium equations in the complex system of Ni(II)-Co(II)-C2O42--NH3-NH4+-H2O at ambient temperature were theoretically deduced. The relationships between lg[Me2+]T and pH at different solution compositions were quantitatively established. At pH<5.0, the metal ion can completely precipitate and the predefined Ni/Co molar ratios in the feed can be maintained in the precursor.
2) The theoretical calculation and experimental results show that free Ni2+ and Co2+ are the dominant metal ions in the solution at pH<5.0, leading to the formation of granular aggregation-shape precursor with the predefined Ni/Co molar ratio in the feed. However, most of Ni2+ and Co2+ coordinate with NH3 to form the soluble complex at pH>5.0, which leads to the formation of rod aggregation-shape precursor in the pH range between 5.0 and 8.0 and fibre-shape precursor at pH>8.0, respectively. The original Ni/Co ratio in the feed and good compositional homogeneity cannot be maintained in the precursors at high pH value (higher than 5.0). The experimental results are consistent with the theoretical analysis.
3) As compared with water medium, the mixture of low surface-tension and viscosity solvent and water used as precipitation medium favors to maintain excellent compositional homogeneity and predefined Ni/Co ratio of the feed in coprecipitation product, and to prepare fibrous precursor at pH<8.6.
References
[1] ZHAN Jing, ZHANG Chuan-fu, LI Chang-jun, WU Lin-lin, CAO Hui, LV Zhan. Status of preparation and application Ni-Co alloy powder [J]. Cemented Carbide, 2002, 19(4): 206-210. (in Chinese)
[2] ZHAN Jing, YUE Jian-feng, ZHANG Chuan-fu. Status of preparation and application of quasi one-dimensional Ni-Co alloy material [J]. Materials Review, 2010, 24(5): 93-96, 100. (in Chinese)
[3] Ghahremaninezhad A, Dolati A. A study on electrochemical growth behavior of the Co-Ni alloy nanowires in anodic aluminum oxide template[J]. Journal of Alloys and Compounds, 2009, 480(2): 275-278.
[4] LIU Qi-ying, GUO Xiao-hui, WANG Tie-jun, LI Yong, SHEN Wei-jie. Synthesi of CoNi nanowire by heterogeneous nucleation in polyol [J]. Mater Lett, 2010, 64(11): 1271-1274.
[5] Zhu Lu-ping, Xiao Hong-mei, Fu Shao-yun. Surfactant-assisted synthesis and characterization of novel chain-like CoNi alloy assemblies [J]. Eur J of Inorg. Chem, 2007, 25: 3947-3951.
[6] Cécile G, Pierrre L, BENEDICTE W F. Electrochemical synthesis of cobalt-nickel nanowires in an ethanol-water bath [J]. Mater Lett, 2008, 62(14): 2106-2108.
[7] Zhang Dong-en, Ni Xiao-min, Zhang Xiao-jun, Zheng Hua-gui. Synthesis and characterization of Ni-Co needle-like alloys in water-in-oil microemulsion [J]. J Magn Magn Mater, 2006, 302(2): 290-293.
[8] Shen Xiang-qian, Cao Kai, Zhou Jian-xin. Preparation of ferromagnetic binary alloy fine fibers by organic gel-thermal reduction process [J]. Trans Nonferrous Met Soc China, 2006, 16(5): 1003-1008.
[9] WU Ming-zai, LIU Guang-qiang, LI Ming-tao, DAI Peng, MA Yong-qing, ZHANG Li-de. Magnetic field-assisted solvothermal assembly of one-dimensional nanostructures of Ni-Co alloy nanoparticles [J]. Journal of Alloys and Compounds, 2010, 491(1-2): 689-693.
[10] HU Ming-jun, LIN Bin, YU Shu-hong. Magnetic field-induced solvothermal synthesis of one-dimensional assemblies of Ni-Co alloy microstructures [J]. Nano Res, 2008, 1(4): 303-313.
[11] ZHANG Chuan-fu, WU Jian-hui, ZHAN Jing, LI Chang-jun, DAI Xi. Precursor synthesis of fibrillar nanocrystalline nickel powder [J]. Nonferrous Metals, 2003, 55(3): 25-29. (in Chinese)
[12] FAN You-qi, ZHANG Chuan-fu, WU Jian-hui, ZHAN Jing, YANG Ping. Composition and morphology of complicated copper oxalate powder [J]. Trans Nonferrous Met Soc China, 2010, 20(1): 165-170.
[13] ZHAN Jing, ZHANG Chuan-fu, LI Tie-jing, WU Jian-hui. Thermodynamic analysis on preparation of fibrous NiO precursor powders with oxalate precipitation process [J]. Trans Nonferrous Met Soc China, 2005, 15(4): 926-930.
[14] ZHANG Chuan-fu, WU Lin-lin, LI Chang-jun, WU Jian-hui. Peparation of fiber precursor of Ni-Co alloy powder [J]. The Chinese Journal of Nonferrous Metals, 2002, 12(1): 182-186. (in Chinese).
[15] PUJOL O, BOWEN P, STADELMANN, HOFMANN. Growth and self-assembly of nanostructure CoC2O4?2H2O particles [J]. J Phys Chem B, 2004, 108(35): 13128-13136.
[16] ZHAN Jing, ZHOU Di-fei, ZHANG Chuan-fu. Shape-controlled synthesis of novel precursor for fibrous Ni-Co alloy powders [J]. Trans Nonferrous Met Soc China, 2011, 21(3):544-551.
[17] DEAN J A. Lange’s handbook of chemistry [M]. Beijing: Science Press, 1991. (in Chinese)
纤维状镍钴合金前驱体制备热力学分析与镍钴配比的控制
湛 菁1, 2,贺跃辉1,周涤非2,张传福2
1. 中南大学 粉末冶金国家重点实验室,长沙 410083;
2. 中南大学 冶金科学与工程学院,长沙 410083
摘 要:根据同时平衡原理和质量守恒原理,推导Ni(II)-Co(II)-C2O42--NH3-NH4+-H2O反应体系中金属离子和草酸盐在常温水溶液中的热力学平衡方程,计算并绘制该体系的lg[Me2+]T—pH 图。结果表明,在水溶液中,当pH值小于5.0时,溶液中镍离子和钴离子以设定配比转移到前驱体粉末中,前驱体的形貌为粒状聚集体;当pH值大于5.0时,由于Ni(NH3)n2+和Co(NH3)n2+ (n=1-6)离子的形成,前驱体粉末中镍钴配比不能维持料液中设定的配比值,前驱体在pH为5.0-8.0范围内呈现棒状形貌,而在 pH>8.0时为纤维状形貌。改变沉淀介质可以在pH为2.0-8.6范围内获得配比准确、成分均匀的纤维状镍钴合金前驱体。
关键词:热力学分析;镍钴合金;草酸镍钴前驱体;设定镍钴配比;纤维状形貌
(Edited by YUAN Sai-qian)
Foundation item: Project (20090162120080) supported by Research Fund for the Doctoral Program of Higher Education of China; Project (20070410989) supported by China Postdoctoral Science Foundation; Project (2010FJ3012) supported by the Program of Science and Technology of Hunan Province, China; Project (09JJ4028) supported by Natural Science Foundation of Hunan Province, China
Corresponding author: ZHOU Di-fei; Tel: +86-731-88836048; E-mail: zdf2011p@163.com
DOI: 10.1016/S1003-6326(11)60834-0
[Me2+]=min{Ksp(MeC2O4(s))/[C2O42-],
[NiNH3]T=[Ni(NH3)2+]+[Ni(NH3)22+]+[Ni(NH3)32+]+
[Ni (NH3)42+]+[Ni(NH3)52+]+[Ni(NH3)62+]
[CoNH3]T=[Co(NH3)2+]+[Co(NH3)22+]+[Co(NH3)32+]+
[Co (NH3)42+] +[Co(NH3)52+]+[Co(NH3)62+]
[NiOH]T=[Ni(OH)+]+[Ni(OH)2(aq)]+[Ni(OH)3-]
[CoOH]T=[Co(OH)+]+[Co(OH)2(aq)]+
[CoOH]T=[Co(OH)+]+[Co(OH)2(aq)]+
[NiC2O4]T=[Ni(C2O4)(aq)]+[Ni(C2O4)22-]+[Ni(C2O4)34-]
[CoC2O4]T=[Co(C2O4)(aq)]+[Co(C2O4)22-]+[Co(C2O4)34-]
[C2O42-]T=[C2O42-]+[HC2O4-]+[H2C2O4]+
[NH3]T=[NH3]+[NH4+]+[NiNH3]T+[CoNH3]T
[Ni2+]T=[Ni2+]+[NiOH]T+[NiC2O4]T+[NiNH3]T
[Co]T=[Co2+]+[CoOH]T+[CoC2O4]T+[CoNH3]T
[Co2+]=min{Ksp(CoC2O4(s))/[C2O42-],
[Ni2+]=min{Ksp(NiC2O4)(s)/[C2O42-],
[17] DEAN J A. Lange’s handbook of chemistry [M]. Beijing: Science Press, 1991. (in Chinese)


