Article ID: 1003-6326(2005)06-1414-06
Thermodynamics of Cu(Ⅱ)-NH3-NH4Cl-H2O system
JU Shao-hua(巨少华), TANG Mo-tang(唐谟堂),
YANG Sheng-hai(杨声海), TANG Chao-bo(唐朝波)
(School of Metallurgical Science and Engineering, Central South University,
Changsha 410083, China)
Abstract:
The thermodynamics of a complex solution system, Cu(Ⅱ)-NH3-NH4Cl-H2O, was presented both theoretically and experimentally. Firstly, according to the principles of simultaneous equilibrium and aqueous electronic charge neutrality of the system, a nonlinear mathematical thermodynamic model with multi-members was set up. In this model, there were six unknowns: pH value, concentration values of free Cl-, free NH3, total concentration values of Cu2+, Cl- and NH3, four equilibrium equations and three equilibrium equations of total quantum of Cu2+, Cl- and NH3, as well as an equilibrium equation of electric charge, were involved in the model. Then after specifying the values of total concentrate of NH3 and Cl-, the model was solved precisely using MATLAB language, and the other four unknowns were obtained. According to the values obtained above, various valuable figures regarding thermodynamic relation of the system were protracted also with MATLAB, including two and three dimensions figures. These figures and data can supply the theoretic conference for optimizing the ratio of leaching reagents in copper extraction through ammonia hydrometallurgy. Finally, the solubility of CuO were measured in the system of NH3-NH4Cl-H2O. The results show that the model and the thermodynamic data obtained are reliable.
Key words:
copper hydrometallurgy; ammoniacal ammonium leaching; metal complex; thermodynamic model; MATLAB language CLC;
number: TQ013.1 Document code: A
1 INTRODUCTION
Ammonia metallurgy has the merits of a high leaching rate, good selectivity, easy purification process, so until now it was still studied in a great area[1-4]. We have previously studied on the thermodynamics in the systems of Zn(Ⅱ)-NH3 -(NH4)2SO4-H2O[5], Zn(Ⅱ)-NH3- (NH4)2CO3-H2O[6] and Zn(Ⅱ)-NH3-NH4Cl-H2O[7] and discovered the rules of zinc solubility in these systems. The former two systems were used to supple the theoretic basis for producing the high grade zinc oxide[8] from zinc oxide materials. While the third system was used to supple the theoretic basis for producing high purity zinc. As for ammonia copper metallurgy, the system of Cu-NH3-H2O has been studied thoroughly. While the system of Cu-NH4Cl-H2O has been studied in the way of construction of φ—pH figure[9]. However, at first, the paper did not discover the rules of the equilibrium solubility of copper in the system; in addition, it supposed the concentration of Cu2+, Cl- and NH3 were certain values.Thus, the results in the study were limited. At last, the method of construction of φ—pH figure is not suitable in studying the system of Cu-NH4Cl-H2O, because the potential of the system is actually a constant, the pH value of this system varies from 6 to 12, and more over, it is very difficult to adjust pH value of the system. Actually, it is more suitable to use the method of construction of the concentrate figure of c(Cu2+)-c(NH4Cl)-c(NH4OH).
As the development of using ammoniacal ammonium chloride metallurgy to treat copper oxide ore[10,11], to clarify the thermodynamics of this system becomes more and more exigent.
In this paper, at first, according to the principles of simultaneous equilibrium and aqueous electronic charge neutrality of the system, a nonlinear mathematical thermodynamic model with multi-members was set up. In addition, the model was solved precisely through programming with MATLAB language. Thirdly, according the values obtained, various valuable figures of thermodynamic relation of the system were protracted also with MATLAB language, and the character of the system was discussed. At last, a series of experiments were carried out to show the reliability of the model and the thermodynamic data.
2 THERMODYNAMIC ANALYSIS AND MODEL CONSTRUCTION
There are altogether 20 species as Cu(NH3)2+, Cu(NH3)2+2, Cu(NH3)2+3, Cu(NH3)2+4, Cu-(NH3)2+5, CuCl+, CuCl2(aq), CuCl-3, CuCl2-4, CuNH3(OH)+, CuNH3(OH)-3, Cu(NH3)2-(OH)2(aq), Cu(OH)2(s), Cu(OH)1.5Cl0.5(s), Cu2+, Cl-, NH3(aq), NH+4, H+, OH-, in the system of Cu(Ⅱ)-NH3-NH4Cl-H2O.
Actually, the species such as CuNH3(OH)+, CuNH3(OH)-3 and Cu(NH3)2(OH)2(aq) could only be produced when the pH value was very high. In this system, the pH value was kept in the range of 6.0 - 12.5. So these species were ignored in this thermodynamic model. While for the species such as CuCl2(aq), CuCl-3 and CuCl2-4, their stable constants were very small in the ammonia systems, so these species were also ignored in our thermodynamic model.
On the base of the simultaneous equilibrium principle, every copper complex is equilibrium with the copper oxide at the present of copper oxide in the system:
CuO+iNH3+H2O-Cu(NH3)2+i+2OH-(1)
CuO+(i-1)H2O=Cu(OH)2-ii+(i-2)H+(2)
CuO+H2O+iCl-=CuCl2-ii+2OH-(3)
According to the exponential computation method[12, 13] and supposing that the activity of each species is equal to the mole concentration of itself, the concentration of these species can be expressed as
c(R)=exp(A+B·pH+C·lnc(NH3(aq))+
D·lnc(Cl-))(4)
where c(R) is every species mole concentration; A is the constant calculated from equilibrium constants or thermodynamic data; B stands for the multiplication of ln10 and gained or lost proton number; C indicates the numbers of ammonia ligand; while D stands for the number of chloride ligand.
The critical stability constants of copper complexes, presented in Table 1, were chosen from Ref.[14]. The thermodynamic data, presented in Table 2, were chosen from Ref.[15].
There are altogether three solid phases in this system, CuO, Cu(OH)2(s) and Cu(OH)1.5Cl0.5(s).
For Cu(OH)2(s), the analytical expression of the equilibrium concentration of Cu2+ is as follows:
c(Cu2+)1=exp(20.9535-4.606·pH)(5)
While for CuO, the analytical expression of the equilibrium concentration of Cu2+ is as follows:
c(Cu2+)2=exp(16.9250-4.606·pH)(6)
Thus, the equilibrium concentration of Cu2+ with Cu(OH)2(s) in this system is always larger than the equilibrium concentration of Cu2+ with CuO. This means that the equilibrium solubility of Cu(OH)2(s) is larger than that of CuO. Put it in another way, in the whole leaching process of CuO, no Cu(OH)2(s) can be presented in this sys-tem. So, in this certain thermodynamic model,
Table 1 Critical stability constants of copper complexs at 298K
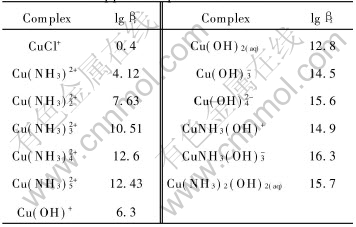
Table 2 Gibbs free energy of related species at 298K (J/mol)
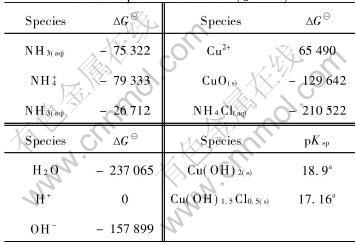
Cu(OH)2(s) was not considered.
While as for Cu(OH)1.5Cl0.5(s), the analytical expression of the equilibrium concentration of Cu2+ is as follows:
c(Cu2+)3=exp(8.8435-3.4545·pH-
0.5·lnc(Cl-))(7)
For compare the size of c(Cu2+)3 and c(Cu2+)2, an inequation is constructed:
lnc(Cu2+)3-lnc(Cu2+)2=-8.0815+
1.1515·pH-0.5·lnc(Cl-)〈0(8)
Thus, when the above inequation (8) is true, the phase of Cu(OH)1.5Cl0.5(s) would exist in the system, while otherwise, when inequation (8) is not true, CuO would be the only solid phase. After solving the inequation, it is found that only when pH〈7.0 and c(Cl-)>2.718, the inequation come into existence. But, the results of solving the model when CuO is the only solid phase showed that only in pure NH4Cl solution can the equilibrium pH value be smaller than 7.0. This means that Cu(OH)1.5Cl0.5(s) would only exist in a very limited range. Thus, for simplifying the process of solving the model, CuO is looked as the only solid phase existing in the system.
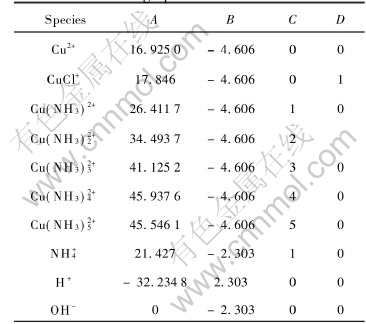
Using the data in Table 2, the values of A, B, C and D in Eqn.(4) can be calculated and listed in Table 3.
Table 3 Constants in exponential Eqn.(1) for calculating species concentration
According to the principle of substance quantity changeless, the sum concentration of copper, ammonia and chloride can be expressed as Eqns.(9), (10) and (11), respectively:
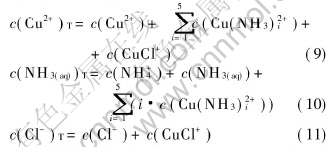
where c(NH3(aq))T is the total concentration of ammonia and ammonium in the system; c(NH3 (aq)) is the concentration of free ammonia in system; i, j and k are the numbers of ammonia, chloride and hydroxide ligands, respectively.
According to the principle of electronic charge neutrality, the equation of electronic charge equilibrium can be expressed as
2·c(Cu2+)T+c(NH4+)+c(H+)=
c(Cl-)T+c(OH-)(12)
Then the model of this system can be set up as a combination of above four Equations, i.e. Eqns.(9) - (12).
3 MODEL SOLVING WITH MATLAB AND RESULTS AND DISCUSSION
The relation among the six variables of c(Cu2+)T, c(Cl-)T, c(NH3(aq))T, c(Cl-), c(NH3(aq)) and pH is confined by the model. If two of them are given, other four variables may be obtained from the above mentioned simultaneous equations by the computation program with MATLAB language compiled by ourselves.
During the actual calculating process, as the total concentration of Cl- and NH3(aq) is determined by the composition of the leaching reagent, so it is preferable to specify these two values, both varying from 0 -5mol/L. The calculated dissolvability of CuO in the system of Cu(Ⅱ)-NH3-NH4Cl-H2O is listed in Table 4.
The calculated results are also shown in Figs.1-5.
By thorough analysis of Fig.1, it shows that: 1) the equilibrium concentration of Cu2+ in a pure ammonia aqueous solution or in a pure ammonium chloride aqueous solution is very low. 2) when the ratio of c(NH4OH) to c(NH4Cl) is lower than 1, the equilibrium concentration of Cu2+ increases rapidly with the increasing ammonia concentration, but when the ratio is larger than 1, the equilibrium concentration of Cu2+ increases slowly with the in-creasing ammonia concentration; 3) when the ratio
Table 4 Calculated dissolvability of CuO in system of Cu(Ⅱ)-NH3-NH4Cl-H2O (mol/L)
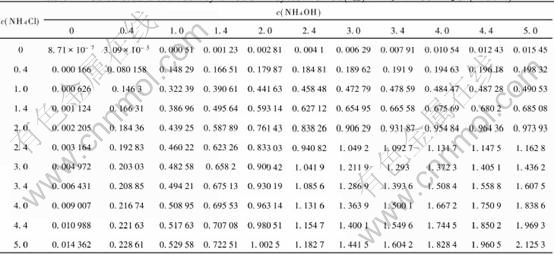
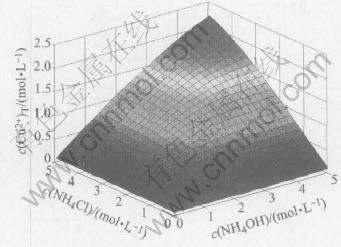
Fig.1 Relationship of c(Cu2+) with c(NH4OH) and c(NH4Cl) in Cu(Ⅱ)-NH3-NH4Cl-H2O system
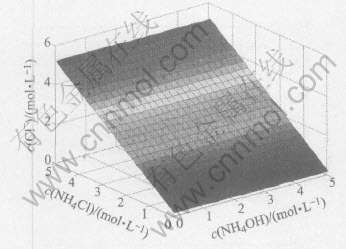
Fig.2 Relationship of c(Cl-) with change of c(NH4Cl) and c(NH3(aq)) in system of Cu(Ⅱ)-NH3-NH4Cl-H2O
Fig.3 Relationship of c(NH3(aq)) with c(NH4OH) and c(NH4Cl) in system of Cu(Ⅱ)-NH3-NH4Cl-H2O
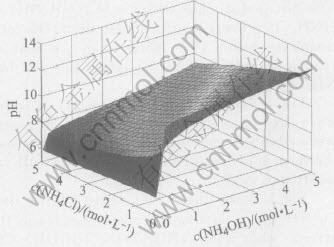
Fig.4 Relationship among pH, c(NH4OH) and c(NH4Cl) in system of Cu(Ⅱ)-NH3-NH4Cl-H2O
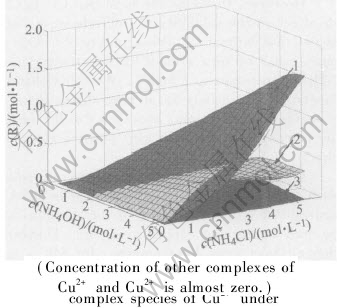
Fig.5 Concentration of three main complex species of Cu2+ under various c(NH4OH) and c(NH4Cl) in system of Cu(Ⅱ)-NH3-NH4Cl-H2O
of c(NH4Cl) to c(NH4OH) is lower than 1, the equilibrium concentration of Cu2+ increases rapidly with increasing ammonium chloride concentration, however, when the ratio is larger than 1, the equilibrium concentration of Cu2+ increases slowly with the increasing ammonium chloride concentration.
From Fig.2 it can be seen that the concentration of free Cl- increases with the increasing ammonium chloride concentration, and the concentration of free Cl- is almost equal to the concentration of initial ammonium chloride. This means that Cu2+ almost doesnt not form complex with Cl- in this system.
Fig.3 shows that when the ratio of c(NH4OH) to c(NH4Cl) is lower than 1, the concentration of free ammonia is almost equal to zero, while when the ratio is larger than 1, the concentration of free ammonia increases rapidly. This means that when the ratio of c(NH4OH) to c(NH4Cl) is lower than 1, almost all of NH3 form complexes with Cu2+. While when the ratio of c(NH4OH) to c(NH4Cl) is larger than 1, free NH3 increases rapidly.
Fig.4 shows that the value of pH increases when the concentration of NH4OH increases, while when the concentration of NH4Cl increases, the value of pH decreases, and the value of pH varies from 6 to 12.5.
Fig.5 shows that: 1) at most of the time Cu(NH3)2+4 is the domain copper complex in the system, while when c(NH4Cl)-c(NH4OH)〈2, Cu(NH3)2+5 becomes the domain copper complex; 2) the sum of the concentration of Cu(NH3)2+3, Cu(NH3)2+4 and Cu(NH3)2+5 have almost more than 99% of all copper complexes.
However, this does not mean that Cl- has little affect on the solubility of CuO, because Fig.1 shows that without Cl- the solubility of CuO is almost equal to zero even if the concentration of NH4OH increases to 5mol/L. We think that the function of Cl- mainly lies in neutralizing electronic charge of the solution, because a Cu(Ⅱ)-ammonia complex always takes two positive electronic charge.
From above we can also find that other anions such as SO2-4, NO-3 or CO2-3 can also be used to neutralizing electronic charge of the solution, and be used in leaching copper oxide ore. However, they all have some disadvantages: for SO2-4, it is precipitable with Ca2+, which is very abundant in the ore, and will be consumed quickly; for NO-3, it is easy to be reduced and volatile; as for CO2-3, it is also precipitable with Ca2+ and Mg2+, and it is also volatile. While Cl- doesnt precipitate with Ca2+ and Mg2+, and its volatility is relatively low. So, ammonium chloride may be the most suitable leaching reagent for copper oxide ore.
4 EXPERIMENTAL CONFORMATION
Excessive copper oxide of the analysis grade was added to the aqueous solution of ammonia and ammonium chloride at a certain concentration, then agitated for 168h at temperature 25℃. Finally the copper concentration of the filtered solution was analyzed. A comparison of the values of c(Cu2+)T of experimental and theoretically calculated is shown in Figs.6-8.
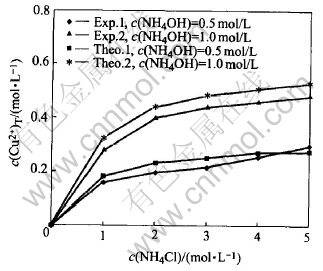
Fig.6 Comparison of experiment c(Cu2+)T with theoretically calculated in system of Cu(Ⅱ)-NH3-NH4Cl-H2O (group 1)
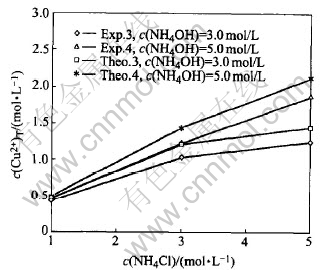
Fig.7 Comparison of experiment c(Cu2+)T with theoretically calculated in system of Cu(Ⅱ)-NH3-NH4Cl-H2O (group 2)
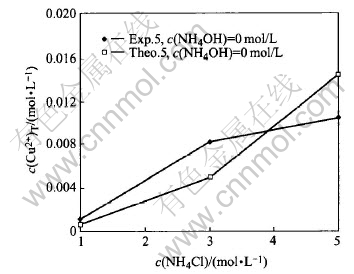
Fig.8 Comparison of experiment c(Cu2+)T with theoretically calculated in system of Cu(Ⅱ)-NH3-NH4Cl-H2O (group 3)
Fig.6 and Fig.7 show that: 1) the experimental values are very similar with the theoretical values under the same condition, the absolute average error between the experimental values and the theoretically calculated values of copper equilibrium concentration is 9.63%; 2) most of the experimental values are smaller than the theoretical values under the same condition, this may be because during experiments, the evaporation of the ammonia could not be prevented completely; 3) under most of the time, the errors between experimental and theoretical values increase when ion strength of the solution increases, this may be due to the decrease of activity of the ions, when ion strength of the solution increases.
Fig.8 shows that when pure ammonium chloride solution is used to dissolve CuO, the errors between the experimental values and theoretical values are somewhat large. Actually, this is because that the new solid phase, Cu(OH)1.5Cl0.5(s), is produced in this case, just as discussed before. From the phenomena of the experiments, it shows that when the concentration of ammonium chloride is higher than 3mol/L, a white or green precipitate, is produced in the conical flask. This effect would decrease the concentration of Cu2+ in the solution. But the second experimental point in Fig.8 is higher than theoretic value. This may be an analysis error occuring here.
5 CONCLUSIONS
1) According to the principle of simultaneous equilibrium and the principle of aqueous electronic charge neutrality, a thermodynamic model of the system, Cu(Ⅱ)-NH3-NH4Cl-H2O, is expressed as Eqns.(9)-(12).
2) Through programming with MATLAB language, the above model is solved precisely and the equilibrium dissolvability of CuO in the system of Cu(Ⅱ)-NH3-NH4Cl-H2O is obtained.
3) Through further analysis with MATLAB language of the above data obtained, it is discovered that ammonia-copper complex, such as Cu(NH3)2+4 and Cu(NH3)2+5, are the predominant species in this system.
REFERENCES
[1]ZHONG Shao-lin, Malcoln T, Hepworth. A calculation method for determining equilibria in metal-ammonia-water systems [J]. Hydrometallurgy, 1995(38): 15-37.
[2]LI De-liang, TANG He, HUANG Nian-dong, et al. A united technique for recovering Au, Ag, Cu from mixed flotation concentrate[J]. The Chinese Journal of Nonferrous Metals, 1999, 9(3): 615-619.(in Chinese)
[3]YU Jing-kai, FANG Zhao-heng. Electrochemical study on anodic oxidation of arsenopyrite in ammonia solutions [J]. The Chinese Journal of Nonferrous Metals, 2000, 10(4): 572-575.(in Chinese)
[4]XU Hui, SU Yuan-zhi, LI Xin-hai, et al. Technology of preparation for light magnesium oxide from bischofite[J]. The Chinese Journal of Nonferrous Metals, 2004, 14(10): 1776-1781.(in Chinese)
[5]TANG Mo-tang, LU Jun-le. On the ammoniation complex equilibria in the system of Zn(Ⅱ)-NH3 -(NH4)2SO4-H2O [J]. J Cent South Inst Min Met, 1994, 25(6): 701-705.(in Chinese)
[6]OUYANG Min. The Study on a New Metallurgical and Chemical Process for Treating Lanpings Zinc Oxide Ores [D]. Changsha: Central South University of Technology, 1994.
[7]YANG Sheng-hai, TAN Mo-tang. Thermodynamics of Zn(Ⅱ)-NH3-NH4Cl-H2O system[J]. Trans Nonferrous Met Soc China, 2000, 10(6): 830-833.
[8]TANG Mo-tang, OU YANG Min. Preparation of graded zinc oxide by using ammonium and ammonia complex leaching process [J]. The Chinese Journal of Nonferrous Metals, 1998, 8(1): 118-121.(in Chinese)
[9]Carmen N, Ignacio G. Thermodynamics of Cu-H2SO4- H2O and Cu-NH4Cl-H2O based on predominance-existence diagrams and Pourbaix-type diagrams[J]. Hydrometallurgy, 1996, 42: 63-82.
[10]WANG Cheng-yan. Exploitation of rebellious low grade copper[J]. Mining & Metallurgy, 2001, 10(4): 49-53.(in Chinese)
[11]WANG Cheng-yan. Solvent extraction study on high alkali and low grade copper oxide ore [J]. Nonferrous Metals (Metallurgy part), 2003(3): 2-7.(in Chinese)
[12]TANG Mo-tang, ZHAO Tian-cong. A thermodynamic study on the basic and negative potential fields of the systems of Sb-S-H2O and Sb-Na-S-H2O[J]. J Cent South Inst Min Met, 1988, 19(1): 35-43.(in Chinese)
[13]TANG Mo-tang, ZHAO Tian-cong, et al. Principle and application of the new chlorination-hydrolization process[J]. J Cent South Inst Min Met, 1992, 23(4): 405-411.(in Chinese)
[14]Smith R M, Martell A E. Critical Stability Constants [M]. New York: Plenum Press, 1976(4).
[15]Pourbaix M. Atlas Dequibres Elextrochimiques et 25℃ [M]. Paris : Publication du Centre Belege dEtude de la Corrosion cebelior, 1963. 407-408.
(Edited by LI Xiang-qun)
Received date: 2005-03-03; Accepted date: 2005-07-04
Correspondence: JU Shao-hua, PhD; Tel: +86-731-8830470; E-mail: shj_200801@sina.com


