Trans. Nonferrous Met. Soc. China 24(2014) 3348-3355
Influence of thermal history on conversion of aluminate species in sodium aluminate solution
Xiao-bin LI, Dong-feng ZHAO, Shuai-shuai YANG, Dan-qin WANG, Qiu-sheng ZHOU, Gui-hua LIU
School of Metallurgy and Environment, Central South University, Changsha 410083, China
Received 28 October 2013; accepted 17 January 2014
Abstract:
It is necessary to clarify the influence of thermal history on the conversion of aluminate species in sodium aluminate solution in order to optimize Bayer alumina production. The interconversion of various solution species in the systems was investigated by measuring the infrared spectra of sodium aluminate solution with different compositions after separate heat treatment, dilution and concentration. The results show that increasing temperature or prolonging holding time favors the transformation of Al2O(OH)62- to Al—OH vibration (condensed AlO4 tetrahedral aluminate ion) at about 880 cm-1 and Al(OH)4-. A12O(OH)62- and Al—OH tetrahedral dimer ions convert rapidly to Al(OH)4- during the dilution process; however, the back transformation of Al(OH)4- to the Al—OH tetrahedral dimer ions can occur in diluted sodium aluminate solution. As for the concentration process, the transformation of Al(OH)4- to A12O(OH)62- and Al—OH tetrahedral dimer ions can take place, while it is relatively difficult to transform to A12O(OH)62-.
Key words:
aluminate ions; structural changes; infrared spectrum; thermal history;
1 Introduction
Sodium aluminate solution is an important carrier of alumina in alumina production process. The Bayer process mainly consists of bauxite digestion, dilution and sedimentation of digested slurry, gibbsite precipitation from purified sodium aluminate solution, concentration of the spent liquor by evaporation. The thermal history of sodium aluminate solution is quite different due to the sodium aluminate solutions with different compositions undergoing different temperatures and residence time. Generally, thermal history has an important effect on the nucleation and metastable zone width of solutions which mainly depend on the ion structure and physical purity [1]. The research shows that the ion structure of sodium aluminate solution affects its physicochemical properties and thus influences the related processes of alumina production [2]. Therefore, it is of great importance to study the influence of thermal history on the conversion of aluminate species in sodium aluminate solutions.
Up to now, many researches on the influence of solution compositions on the ion structure of sodium aluminate solution have been published. The existence of dominant monomeric tetrahydroxy aluminate ions (Al(OH)4-) and Al(III)-containing dimers (e.g. Al2O(OH)62- and Al2(OH)82-) as minor species in synthetic Bayer liquors has been well established [3-7]. Furthermore, the distribution of aluminate species is affected by solution compositions. Aluminate ions in sodium aluminate solution with low concentration and caustic ratio (molar ratio of Na2O to Al2O3, expressed as αk) are dominantly in the form of monomers (Al(OH)4-), and the amount of Al(OH)4- reduces while the amount of Al2O(OH)62- increases correspondingly with increasing solution concentration and caustic molar ratio. A small quantity of Al(OH)63- appears only in the solution with high concentration and caustic ratio [8]. The influence of impurities on the ion structure was also reported in Ref. [9]. Although the influence of solution compositions on the ion structure has been well understood, the effects of thermal history on the ion structure remain ambiguous with no definite description. ZHANG et al [10] measured the ion structure of sodium aluminate solution obtained by bauxite digestion at 255 and 130 °C, respectively, and the results showed that the dominant aluminate anion is Al(OH)4- for the digestion temperature of 255 °C while polymerized aluminate ions and Al(OH)4- coexist for the digestion temperature of 130 °C. CHEN et al [11] examined the change of aluminate species of the sodium aluminate solution prepared by dissolving aluminum in sodium hydroxide aqueous solution and then placing at room temperature for various time. The aluminate ion structure change of the sodium aluminate solution treated by magnetic field and then placed for different time was also observed in Ref. [12]. The facts mentioned on indicate that the ion structures of aluminate solutions are affected by the thermal history of solutions. Unfortunately, to our best knowledge, no systematic and deep investigations about the variation of various Al(III)-containing species during the thermal treatment process were not yet fully understood till now. Under this consideration, a detailed study was done on aluminate species conversion behavior of aluminate solutions with different compositions treated at different temperatures for various time, with the attempt to clarify the influence of thermal history on aluminate species transformation and ultimately to provide theoretical guidance for Bayer alumina production.
2 Experimental
2.1 Preparation of sodium aluminate solution
According to the preset composition of the solution, the pre-weighed aluminum hydroxide and sodium hydroxide (analytical reagents, Kermel Chemical Reagent Corporation of Tianjin, China) were added into deionized water, stirred and boiled for certain time till the aluminum hydroxide was dissolved completely. The as-prepared aluminate solution was then filtered to remove the fine solids and the filtrate was kept in a sealed polyethylene bottle for the experimental use.
2.2 Spectroscopy analyses of sodium aluminate solutions
The IR spectra of thin films of aluminate solutions between KBr plates were collected on a FT-IR 6700 spectrometer with 4 cm-1 resolution (manufactured by Nicolet Co., USA). The reliability and the repeatability of the potassium bromide (KBr) pellet technique for measuring the aluminate solution were established [2].
Infrared measurements at different temperature were performed using an attachment (HC-32 Heated/ Cooled Transmission Cell) when solution temperature was below 100 °C. When solution temperature was in the range of 100-140 °C and 160-260 °C, the solutions were respectively kept in self-made DY-8 low-pressure group autoclave (with the maximum temperature less than 140 °C and the temperature accuracy of ±1 °C) and XYF-d44×6 self-made high-pressure group autoclave (with the maximum temperature of 350 °C and the temperature accuracy of ±1 °C) at preset temperatures for a certain time, and then their infrared spectra were collected immediately by the coating method after the solution being cooled abruptly. The infrared spectrum of the solution after keeping at a constant temperature of 90 °C in low-pressure group autoclave by the coating method is almost the same with that obtained by the online heated/cooled transmission cell at the same temperature, indicating that the infrared spectra of sodium aluminate solutions obtained by the above method are credible.
3 Results and discussion
3.1 Influence of temperature on conversion of aluminate species in sodium aluminate solution
In alumina production process, different unit operations correspond to different temperatures which fluctuate greatly. Thus it is necessary to study the influence of temperature on the conversion of aluminate species in sodium aluminate solution. In view of this, sodium aluminate solutions with different concentrations and caustic ratio were held at different temperatures for 24 h, then the IR spectra were measured.
The infrared spectra of the sodium aluminate solutions with low caustic molar ratio and medium concentrations after being kept at different temperatures are shown in Fig. 1. As shown in Fig. 1, there are characteristic absorption peaks at 550, 635, 720 and 880 cm-1 in the IR spectra of sodium aluminate solutions when the solutions are held at 25-250 °C with low caustic ratio and caustic soda concentration (as ρ(Na2O)) less than 170 g/L. It is generally known that 550 cm-1 corresponds to the Al—O—Al vibration bands (Al2O(OH)62-), 635 cm-1 and 720 cm-1 correspond to Al—OH symmetry and anti-symmetry stretching bands, respectively (both corresponding to Al(OH)4-). However, it is still uncertain about the vibration mode of the 880 cm-1 band. At present, the dominant view about this is that 880 cm-1 corresponds to the Al—OH vibration (condensed AlO4 tetrahedral aluminate ion). As shown previously [2,3,13-17], 635, 720 and 880 cm-1 bands are all assigned to Al—OH tetrahedral structure.
Moreover, the absorption peak intensity at 720 cm-1 is much greater than that at other bands, suggesting that Al(OH)4- is the main aluminate ion in the sodium aluminate solution. Additionally, a broad weak absorption peak is observed at 550 cm-1 corresponding to Al—O—Al vibration bands of A12O(OH)62- [3,8], indicating that there is a small quantity of A12O(OH)62– in the solution. By comparing each curve in Fig. 1, it can also show that the conversion of aluminate species in sodium aluminate solution is little affected by the temperature with almost no change in the characteristic absorption peaks, when the solution temperature is below 130 °C. However, the absorption peaks at 720 and 880 cm-1 are enhanced while the peaks at 550 cm-1 are slightly weakened with the increase of temperature when solution temperature is greater than 130 °C, indicating that increasing solution temperature can promote the conversion of the complex A12O(OH)62- ions to Al—OH tetrahedral ions as the thermal motion of ions become intensive.
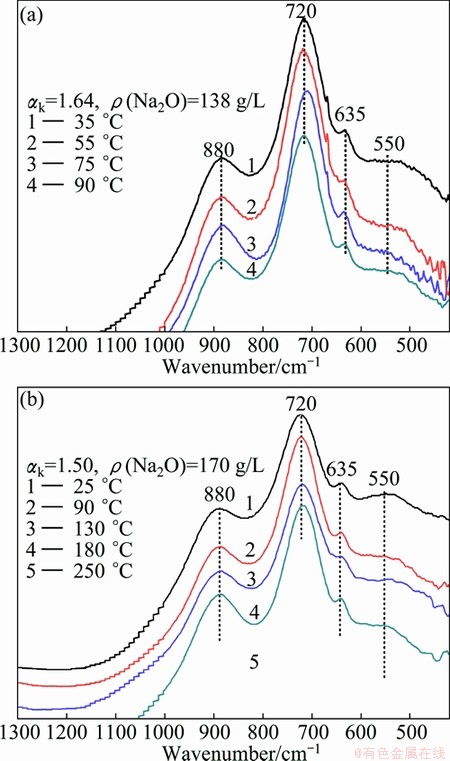
Fig. 1 Influence of temperature on infrared spectra of sodium aluminate solution with different medium concentrations and low caustic ratios
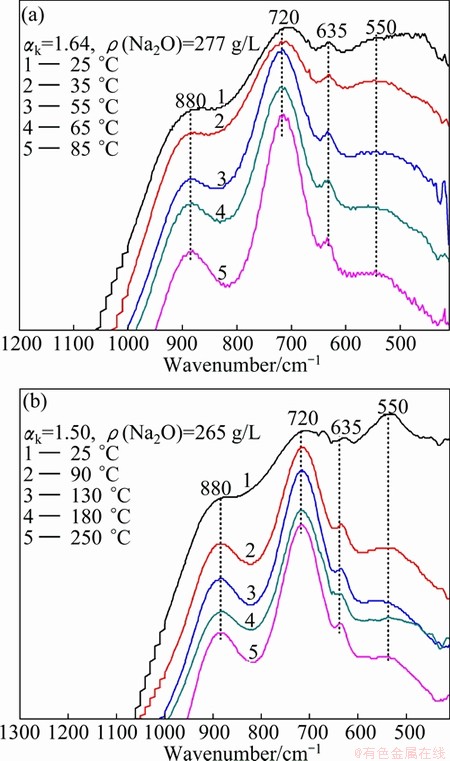
Fig. 2 Effect of temperature on infrared spectra of sodium aluminate solution with different high concentrations and low caustic ratios
The IR spectra of sodium aluminate solutions with low caustic ratio and high caustic concentration (ρ(Na2O) of 270 g/L) held at different temperatures for 24 h were measured, and the results are shown in Fig. 2. The obvious difference of IR spectra of the solutions held at 25 °C in Figs. 2(a) and (b) is due to the different compositions of the solutions. Curve 1 in Fig. 2 represents the infrared spectra of the sodium aluminate solution held at 25 °C with the main absorption peaks appearing at 550, 720 and 880 cm-1. Compared with the peak intensity at 880 cm-1 of curve 1 in Fig. 1, the conclusion can be made that the amount of the tetrahedral aluminate dimer ions at about 880 cm-1 in relatively concentrated aluminate solution reduces due to the dehydration with respect to the solutions with low caustic molar ratio at low temperature. Figure 2 also shows that the characteristic absorption peaks at 720 and 880 cm-1 are intensified gradually and the symmetry become better, while the characteristic absorption peaks at 550 cm-1 are weakened gradually with increasing the temperature. The possible reason is that the number of free water molecules in the solution relatively increases with the rise of temperature, resulting in the following conversion of aluminate ions [18]:
Al2O(OH)62-+H2O Al(OH)4- (1)
Al(OH)4- (1)
Al2O(OH)62-+3H2O [Al2(OH)8(H2O)2]2- (2)
[Al2(OH)8(H2O)2]2- (2)
Figure 3 shows the infrared spectra of sodium aluminate solutions held at different temperatures with different caustic concentrations and caustic ratio of 2.51. It can be known that the infrared spectra change little with the increase of temperature whether ρ(Na2O) is 180 g/L or 350 g/L. This may be attributed to the large number of free OH- ions in sodium aluminate solution with relatively high caustic ratio, where the structure of the aluminate ions are mainly affected by OH- and the influence of temperature gets weaker.
3.2 Influence of holding time on conversion of aluminate species in sodium aluminate solution
In order to learn more about the conversion rules among aluminate species with time, the variation of infrared spectra of sodium aluminate solution with different compositions with the holding time at different temperatures was studied. The results are shown in Fig. 4.
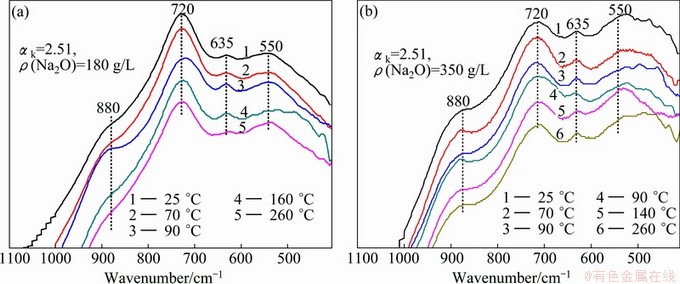
Fig. 3 Effect of temperature on infrared spectra of sodium aluminate solution with different high caustic ratios

Fig. 4 Variation of infrared spectra of sodium aluminate solution with different holding time
Figures 4(a)-(c) show the IR spectra of sodium aluminate solutions with low caustic ratio held at 100, 120 and 260 °C for different time, respectively. Curve 1 (0 h) shows the infrared spectrum of sodium aluminate solution without additional thermal treatment, with the major absorption peaks at 550, 635, 720 and 880 cm-1. The IR spectrum of curve 1 in Fig. 4(a) slightly differs from that of curve 1 in Fig. 4(c) because of different compositions of solutions. With the increase of the holding time, the absorption peaks at 550 cm-1 are gradually weakened, while the peaks at 720 and 880 cm-1 are continuously enhanced with better symmetry. This suggests that the conversion degree of the Al—O—Al vibration of the dimer A12O(OH)62- to the Al—OH vibration of the tetrahedral aluminate ions (Al(OH)4- and [Al2(OH)8(H2O)2]2-) is enhanced with the increase of the holding time. It is also found that the strength and symmetry of absorption peaks at 635, 720 and 880 cm-1 in the IR spectra of the solutions held at 100, 120 and 260 °C tend to be stable after 4, 2 and 1 h, respectively, and the variation of the absorption peak at 550 cm-1 has approximately the same trend. It shows that the conversion of aluminate species in sodium aluminate solutions accelerates as the temperature increases. And curve 1 in Fig. 4(d) shows that the infrared spectra absorption peaks of aluminate solution with high caustic ratio mainly appear at 550 and 720 cm-1, and holding time has no obvious effect on the infrared spectra at holding temperature of 260 °C. By comparing Figs. 4(d) with (c), it can be concluded that thermal treatment of the solution by holding at a constant temperature for certain time has great effect on the conversion of aluminate species for solutions with low caustic ratio but small effect for solutions with high caustic ratio, being in consistence with the results in section 3.1.
3.3 Influence of dilution process on conversion of aluminate species in sodium aluminate solution
Before the seeded precipitation of the purified aluminate solution in Bayer alumina production, dilution and solid-liquid separation of the digested slurry must be carried out. So it is important to study the conversion law among aluminate species in solution of low caustic ratio with the variation of concentration and time during the dilution process. Therefore, high concentrated sodium aluminate solutions were diluted to different concentrations, and their IR spectra were then measured by the coating method. The boiling high concentrated sodium aluminate solution was diluted by adding slowly the boiling distilled water with agitation. Both the composition analysis and the clarity observation show that this dilution operation can avoid gibbsite precipitation from the solution.
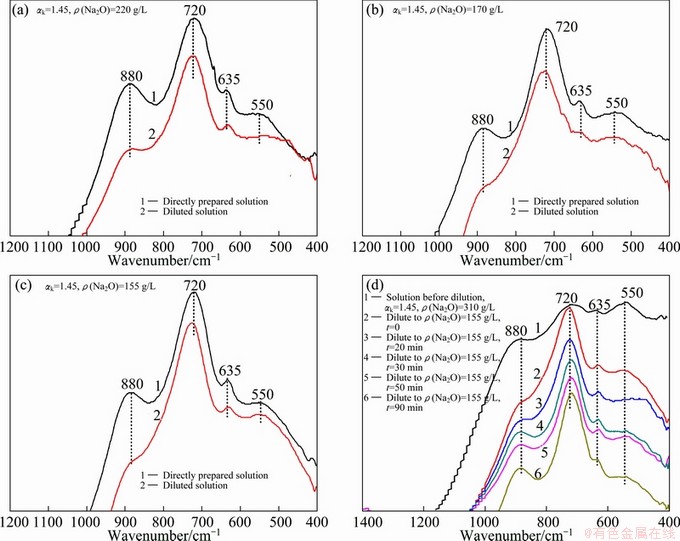
Fig. 5 Influence of dilution on infrared spectra of sodium aluminate solution prepared by different ways
The infrared spectra of sodium aluminate solutions prepared by different ways are shown in Fig. 5. Curves 1 in Figs. 5(a)-(c) correspond to the IR spectra of solutions prepared by directly dissolving aluminum trihydroxide in sodium hydroxide aqueous solution and subsequently placed at room temperature for a long holding time (>2 d), and curves 2 in Figs. 5(a)-(c) correspond to the IR spectra of solutions prepared by diluting the high concentrated solution (ρ(Na2O) of 312 g/L) with distilled water to the same composition as that of curves 1 in Figs. 5(a)-(c) with no holding time after dilution. The comparison between curves 1 and curves 2 in Figs. 5(a)-(c) shows that: 1) the IR spectra absorption peaks of the diluted solutions at 550 cm-1 decrease and this trend becomes more obvious with the increase of concentration; 2) the intensities of the characteristic peaks at 880 cm-1 of curves 2 representing condensed AlO4 tetrahedral aluminate ion reduce remarkably, and the characteristic peaks at 720 cm-1 representing Al(OH)4- are slightly enhanced and shift to high wavenumber. The greater the dilution degree of the solution is, the more obvious the change trend of IR spectra becomes. Figure 5(d) shows the infrared spectra of sodium aluminate solutions diluted and then placed at room temperature for different holding time t, from which it can be seen that absorption peaks at 550 cm-1 change little with the holding time, suggesting that the conversion of A12O(OH)62- is relatively slow. As for the absorption peaks at 880 cm-1, they are revealed gradually with the holding time, and they are close to that of the sodium aluminate solution prepared by the above direct dissolution method after being placed for 90 min (see curve 1 in Fig. 5(c)). The reason may be that condensed AlO4 tetrahedral aluminate ion ( [Al2(OH)8(H2O)2]2-) in the solution is dissociated into hydrated Al(OH)4- ion ([Al(OH)4(H2O)2]- [7] according to the following reaction under the action of water added during the dilution of the solution, then the inverse reaction of the above process occurs, ultimately resulting in the equilibrium distribution of the ions in the solution.
[Al2(OH)8(H2O)2]2-+2H2O 2[Al(OH)4(H2O)2]- (3)
2[Al(OH)4(H2O)2]- (3)
3.4 Influence of concentration process on conversion of aluminate species in sodium aluminate solution
Concentration is the inverse process of dilution. The process of concentration in alumina production includes self-evaporation of digested slurry and evaporation of the spent liquor. Understanding the conversion of aluminate species in concentration process is an important part of the study on thermal history of sodium aluminate solution. For this purpose, concentrated sodium aluminate solutions with different composition were prepared and placed at room temperature for a relatively long time (>2 d), then diluted to different concentrations according to the dilution method in section 3.3, and finally the diluted solutions were concentrated immediately by continuous rapid evaporation in the boiling state. The whole process of dilution and concentration was completed within 40 min. During the operation of dilution and concentration, aluminate samples were taken out and then measured immediately by the infrared spectra by the coating method (as shown in Fig. 6).
Figure 6 shows that solution concentration increases with the evaporation processing, and the absorption peaks at 550 cm-1 are enhanced while the peaks at 720 cm-1 are weakened, revealing that Al(OH)4- converts gradually to A12O(OH)62-, which is in agreement with the influence rule of the solution composition on the conversion of aluminate species. For the solutions with low caustic ratio, the shape of the IR spectrum absorption peak at 880 cm-1 of the concentrated solution is generally the same with that of the diluted solution with the same concentration; however, the absorption peaks at 550 cm-1 cannot be restored, as shown in Fig. 6(a). As the caustic ratio increases, the shapes of the IR spectra absorption peaks at 880 cm-1 and 550 cm-1 of the concentrated and diluted solutions are essentially the same, as shown in Figs. 6(b) and (c). It indicates that the dilution of the solution with low caustic ratio is in favor of the A12O(OH)62- transformation and the concentration process is difficult to make it recover, while the concentration process of the solution with high caustic ratio can restore this transformation. It also indicates that the conversion of the tetrahedral aluminate ions at about 880 cm-1 is relatively fast, and it can recover through concentration process of solutions with either low caustic ratio or high caustic ratio.
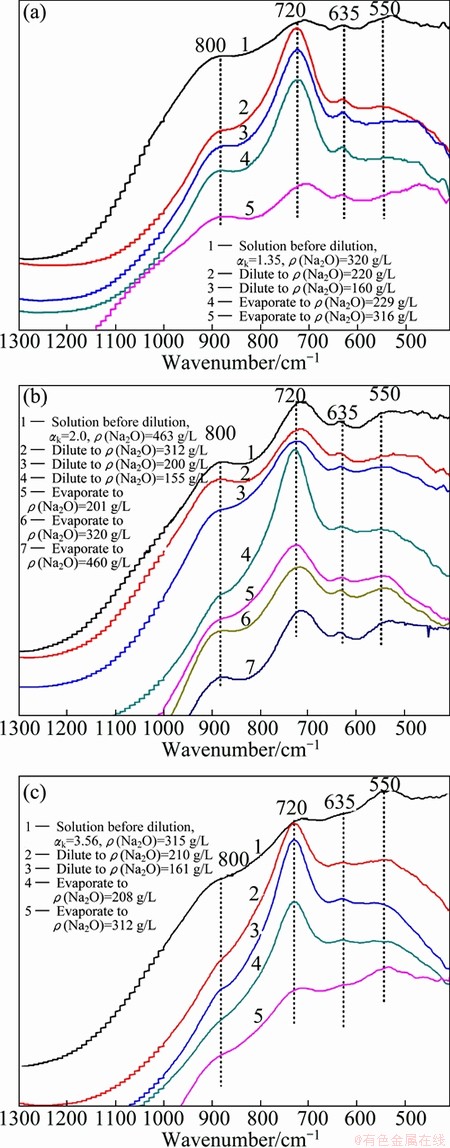
Fig. 6 Infrared spectra change of sodium aluminate solution during dilution and evaporation process
4 Conclusions
1) Increasing temperature favors the conversion of Al2O(OH)62- to Al(OH)4- and the Al—OH tetrahedral dimer ions at about 880 cm-1; moreover, the conversion becomes great with increasing the solution concentration, decreasing the caustic ratio of the solution and extending the holding time.
2) The dilution process promotes the conversion of A12O(OH)62- and the Al—OH tetrahedral dimer ions to Al(OH)4- in sodium aluminate solution, and the conversion is more obvious with increasing the dilution degree. In addition, the reconversion of Al(OH)4- to the tetrahedral aluminate ions at about 880 cm-1 in the diluted solution can occur while the amount of A12O(OH)62- does not change.
3) The concentration process benefits the conversion of Al(OH)4- into A12O(OH)62- and the Al—OH dimer ions, and the conversion degree gets great with increasing the caustic molar ratio of the solution. But it is relatively difficult to convert to A12O(OH)62- for solutions with low caustic ratio.
Acknowledgements
The authors gratefully acknowledge our colleagues Prof. Zhi-hong PENG and Dr. Tian-gui QI for their support during the experiments.
References
[1]  The kinetics of industrial crystallization [M]. New York: Elsevier, 1985.
The kinetics of industrial crystallization [M]. New York: Elsevier, 1985.
[2] LI Xiao-bin, WANG Dan-qin, ZHOU Qiu-sheng, LIU Gui-hua, PENG Zhi-hong. Concentration variation of aluminate ions during the seeded precipitation process of gibbsite from sodium aluminate solution [J]. Hydrometallurgy, 2011, 106(1): 93-98.
[3] Moolenaar R J, Evans J C, McKeever L D. Structure of the aluminate ion in solutions at high pH [J]. The Journal of Physical Chemistry, 1970,74(20): 3629-3636.
[4] Radnai T, May M P, Hefter T G, Sipos P. Structure of aqueous sodium aluminate solutions: A solution X-ray diffraction study [J]. The Journal of Physical Chemistry A, 1998, 102(40): 7841-7850.
[5] Sipos P, May P M, Hefter G. Quantitative determination of an aluminate dimer in concentrated alkaline aluminate solutions by Raman spectroscopy [J]. Dalton Transactions, 2006, 2: 368-375.
[6] Watling H. Spectroscopy of concentrated sodium aluminate solution [J]. Applied Spectroscopy, 1998, 52(2): 250-258.
[7] Zambo J. Structure of sodium aluminate liquors: Molecular model of the mechanism of their decomposition [J]. Light Metals, 1986, 2: 199-215.
[8] CHEN Nian-yi, LIU Miao-xiu, CAO Yi-lin, TANG Bo, HONG Mei. Studies on the anionic species of sodium aluminate solutions [J]. Science in China: Series B, 1993, 36(1): 32-38.
[9] WANG Dan-qin, LI Xiao-bin, ZHOU Qiu-sheng, LIU Gui-hua, PENG Zhi-hong. Effect of sodium carbonate and sodium sulfate on structure of sodium aluminate solution [J]. Journal of Central South University: Science and Technology, 2012, 43(12): 4600-4604. (in Chinese)
[10] ZHANG Shao-yun, LIU Yun-qing, CHEN Qi-yuan, MA Jian-ye. Effect of dissolving-out temperature and pressure on structure of sodium aluminate solution [J]. Nonferrous Metals: Extractive Metallurgy, 2011, 1: 16-19. (in Chinese)
[11] Chen Nian-yi, LIU Miao-xiu, YANG Jin-xiu. Influence of the preparative history on physico-chemical properties of sodium aluminate solutions [J]. Journal of Materials Science & Technology, 1992, 8(2): 135-137.
[12] LI Xiao-bin, WANG Dan-qin, ZHOU Qiu-sheng, LIU Gui-hua, PENG Zhi-hong. Influence of magnetic field on the seeded precipitation of gibbsite from sodium aluminate solution [J]. Minerals Engineering, 2012, 32: 12-18.
[13] Carreira L A, Maroni V A, SwaineJr J W, Plumb R C. Raman and infrared spectra and structure of the aluminate ions [J]. The Journal of Chemical Physics, 1966, 45(6): 2216-2220.
[14] Chen Qi-yuan, Li Jie, Yin Zhou-lan, Zhang Ping-min. Decomposition of supersaturated sodium aluminate solution [J]. Transactions of Nonferrous Metals Society of China, 2003, 13(3): 649-655.
[15] Lippincott E R, Psellos J A, Tobin M C. The Raman spectra and structures of aluminate and zincate ions [J]. The Journal of Chemical Physics, 1952, 20(3): 536-536.
[16] Tarte P. Infra-red spectra of inorganic aluminates and characteristic vibrational frequencies of AlO4 tetrahedra and AlO6 octahedra [J]. Spectrochimica Acta Part A: Molecular Spectroscopy, 1967, 23(7): 2127-2143.
[17] Sipos P. The structure of Al(III) in strongly alkaline aluminate solutions—A review [J]. Journal of Molecular Liquids, 2009, 146: 1-14.
[18] LI Xiao-bin, WANG Dan-qin, LIANG Shuang, LIU Gui-hua, PENG Zhi-hong, ZHOU Qiu-sheng. Relationship between conductivity and solution structure of sodium aluminate solution [J]. Chemical Journal of Chinese Universities, 2010, 31(8): 1651-1655 (in Chinese).
热历史对铝酸钠溶液中含铝离子之间转化的影响
李小斌,赵东峰,杨帅帅,王丹琴,周秋生,刘桂华
中南大学 冶金与环境学院,长沙 410083
摘 要:为了优化拜耳法生产氧化铝的过程,有必要明晰热历史对铝酸钠溶液中含铝离子间转化的影响。通过对不同组成铝酸钠溶液经过热处理、稀释和浓缩后其红外谱图的测定,研究溶液中含铝离子间的转化规律。结果表明:升高温度和延长保温时间有助于Al2O(OH)62-离子向880 cm-1波数处Al—OH结构四面体聚合物和Al(OH)4-转化;稀释过程中快速发生A12O(OH)62-和Al—OH结构四面体聚合物向Al(OH)4-的转化,但稀释后溶液可发生Al(OH)4-向Al—OH结构四面体聚合物的恢复性转化;浓缩过程则发生Al(OH)4-向Al—OH结构四面体聚合物和A12O(OH)62-的转化,且向A12O(OH)62-的转化较困难。
关键词:铝酸根离子;结构变化;红外光谱;热历史
(Edited by Chao WANG)
Foundation item: Project (51274243) supported by the National Natural Science Foundation of China
Corresponding author: Dan-qin WANG; Tel/Fax: +86-731-88830453; E-mail: wdanqin@csu.edu.cn
DOI: 10.1016/S1003-6326(14)63476-2
Abstract: It is necessary to clarify the influence of thermal history on the conversion of aluminate species in sodium aluminate solution in order to optimize Bayer alumina production. The interconversion of various solution species in the systems was investigated by measuring the infrared spectra of sodium aluminate solution with different compositions after separate heat treatment, dilution and concentration. The results show that increasing temperature or prolonging holding time favors the transformation of Al2O(OH)62- to Al—OH vibration (condensed AlO4 tetrahedral aluminate ion) at about 880 cm-1 and Al(OH)4-. A12O(OH)62- and Al—OH tetrahedral dimer ions convert rapidly to Al(OH)4- during the dilution process; however, the back transformation of Al(OH)4- to the Al—OH tetrahedral dimer ions can occur in diluted sodium aluminate solution. As for the concentration process, the transformation of Al(OH)4- to A12O(OH)62- and Al—OH tetrahedral dimer ions can take place, while it is relatively difficult to transform to A12O(OH)62-.


