文章编号:1004-0609(2015)-09-2604-08
木屑季铵螯合吸附剂的制备及其吸附铀矿酸法废水中U(VI)
邓文静1,周书葵1, 2,刘迎九1,曾光明2,江海浩1,康 丽1,方 良1
(1. 南华大学 城市建设学院,衡阳 421001;
2. 湖南大学 环境科学与工程学院,长沙 410082)
摘 要:
针对铀矿中酸法含铀废水处理的难点,利用木屑制备季铵盐型螯合吸附剂(MS)强化其除铀效果。研究环氧氯丙烷(ECH)添加量、二乙烯三胺(DETA)添加量、醚化反应时间、接枝反应时间对木屑的改性效果及其对废水中U(VI)吸附效果的影响,获取制备MS的最佳工艺条件。对MS进行表征分析并探讨吸附剂的改性机理。单因素实验结果表明:随着ECH、DETA添加量的增加及醚化时间的延长,MS对U(VI)的吸附性能先增大后减小;而随着接枝时间的增加,MS对U(VI)的吸附效果先增大而后基本保持不变。正交优化实验结果表明:对U(VI)吸附性能的影响醚化反应时间最大,接枝反应时间次之,ECH、DETA添加量最小。最优制备条件为ECH添加量10 mL,DETA添加量6 mL,醚化反应时间1 h,接枝反应时间4 h,此时MS对U(VI)的吸附率为99.72%,吸附量达99.72 mg/g。
关键词:
中图分类号:O636.2;X591 文献标志码:A
Preparation of quaternary ammonium salt modified sawdust chelate adsorbent and its U(VI) adsorption in wastewater from uranium milling plant
DENG Wen-jing1, ZHOU Shu-kui1, 2, LIU Ying-jiu1, ZENG Guang-ming2,
JIANG Hai-hao1, KANG Li1, FANG Liang1
(1.School of Urban Construction, University of South China, Hengyang 421001, China;
2. School of Environment Science and Engineering, Hunan University, Changsha 410082, China)
Abstract: The problem of processing uranium wastewater in the uranium milling plant was studied by preparing a kind of quaternary ammonium salt chelate adsorbent (MS) to strengthen the effect of adsorbing uranium. The effects of epichlorohydrin dosage, diethylenetriamine dosage, etherification reaction time, grafting reaction time on the modification effect of sawdust and its U(VI) adsorption in wastewater were studied, and the optimal technological conditions for preparing MS were gotten. And the MS was characterized and the modification mechanism of the adsorbent was studied. The single factor test result shows that with the increase of ECH dosage, DETA dosage and etherification reaction time, U(VI) adsorption increases and then decreases, and with the increase of grafting reaction time, U(VI) adsorption increases and then keeps unchanged. The orthogonal test result shows that the largest effect for U(VI) adsorption is etherification reaction time, then grafting reaction time, and the smallest effect for U(VI) adsorption is ECH and DETA dosage. The technological parameters optimized by conducting orthogonal test for the U(VI) adsorption are epichlorohydrin dosage of 10 mL, diethylenetriamine dosage of 6 mL, etherification reaction time of 1 h, grafting reaction time of 4 h. Under this condition, the highest adsorption rate is 99.72% and the highest adsorption capacity is 99.72 mg/g.
Key words: sawdust; quaternary ammonium salt; U(VI); adsorption
随着核工业技术和设施的不断进步,铀的需求量也愈来愈大,同时在采冶过程产生大量含铀废水[1]。铀因其在低浓度和长时间下的化学毒性和放射性而危害环境和人体健康[2]。美国环保局(USEPA)将铀归类为一种确定的致癌物质并表明零容忍是唯一安全的可接受极限[3]。去除废水中的铀仍是一个极具挑战的难题[4]。
目前处理含铀废水的方法众多[5-7],各有其优劣,但吸附法去除效率高、工艺简单、成本低[8],且吸附后的吸附剂处理简单、危害较小(焚烧或热处理)[9]。木屑吸附剂具备成本低、材料量大、易获取、环境友好及无二次污染等特点,但直接应用时吸附率不高,因此可通过改性提高其吸附率[10]。胺类螯合吸附剂与重金属离子结合,形成较稳定的配合物[11-12],具有去除程度高、吸附选择性好、吸附速度快,受碱金属、碱土金属影响小等一系列优势而备受关注[13-14]。除此之外,季铵盐型吸附剂因其季铵盐基团而增强吸附速率,化学及酸碱稳定性,且它是一种螯合吸附剂的同时也是一种阴阳离子吸附剂[15]。PADMAVATHI等[16]分别用季铵化和聚苯乙烯-乙烯-丁烯聚砜膜吸附工业废水中的重金属离子Cu2+、Ni2+和Cr6+,发现季铵化吸附剂吸附效果较好。曹威等[17]用季铵化稻草来吸附水中的SO42-,其最大单分子层吸附量为74.76 mg/g,远大于原稻草的吸附容量(11.68 mg/g)。目前,关于螯合吸附剂对铀吸附的研究主要集中在制备偕胺肟化纤维等方面,但制备时间及饱和吸附时间较长,重复使用效果不佳,且由于笼蔽效应和纤维上吸附的铀酰离子的排斥作用使其吸附能力不强[18]。利用木屑合成季铵盐型螯合吸附剂材料解决上述问题却鲜见文献报道。
本文作者通过化学改性制备季铵盐型螯合吸附剂并深入研究其改性机理,将U(VI)的吸附率作为确定最佳制备条件的指标,并将其作为实际应用的参考。
1 实验
1.1 实验材料
废水来源为模拟中国南部某铀矿酸法含U(VI)废水,其质量浓度为10 mg/L,主要组成为UO22+。主要试剂有氢氧化钠(NaOH,分析纯),环氧氯丙烷(ECH,分析纯),N,N-二甲基甲酰胺(DMF,分析纯),二乙烯三胺(DETA,分析纯),三乙胺(TEA,分析纯),U3O8(基准纯,国家标准物资中心生产)。
铀的测定方法为水中微量铀分析方法(GB6768—86)[19]—分光光度法。
1.2 季铵盐型螯合吸附剂的制备
将木屑粉碎过孔径为250 μm筛,自来水清洗后用蒸馏水冲洗干净,80 ℃条件下于干燥箱中烘干至恒重后得预处理木屑样品RS,存于干燥器中备用。
将2 g RS放入三口圆底烧瓶中经质量分数为30%的NaOH溶液在80 ℃的恒温水浴锅中处理1 h[20-21]后,加入0~16 mL氧氯丙烷和8 mL N,N-二甲基甲酰胺[22],在100 ℃的条件下反应0~2 h(醚化反应过程),加入0~20 mL二乙烯三胺在100 ℃的条件下反应1 h(交联反应过程),再加入8 mL三乙胺[23]在100 ℃的条件下反应0~4 h(接枝反应过程),多次清洗至中性,经真空泵压滤,在80 ℃下于干燥箱中干燥12 h得季铵盐型螯合吸附剂MS。
1.3 改性木屑结构表征
采用Ultima IV型X射线衍射仪(日本Rigku公司生产)测定样品的XRD图谱;采用Scientific型傅里叶变换红外吸收光谱仪(美国Thermo公司生产)分析样品的红外光谱;采用S4800型扫描电镜(日本Hitachi公司生产)表征样品显微组织。
1.4 含铀废水吸附处理实验
取pH为5,质量浓度为10 mg/L的铀溶液100 mL置于锥形瓶中,再加入0.01 g改性木屑,于恒温摇床中在25 ℃、150 r/min条件下振荡3 h,过滤后测定滤液中残余铀质量浓度,并按式(1)和(2)计算改性木屑对铀的吸附率R及吸附容量qt[24]:
R=[(c0-ce)/c0]×100% (1)
qt=[(c0-ce)V]/m (2)
式中:c0为铀的初始质量浓度,mg/L;ce为铀的平衡质量浓度,mg/L;V为U(VI)溶液的总体积,mL;m为吸附剂的添加量,mg。
1.5 正交试验设计
为优化实验结果,在单因素实验结果的基础上,用正交合成实验来研究ECH添加量、DETA添加量、醚化反应时间和接枝反应时间4个主要因素对吸附效果的影响,从而确定最佳制备方案。
2 结果与分析
2.1 形貌分析
图1所示为木屑改性前后的SEM像。由图1可看出,改性前木屑(RS)表面粗糙、结构紧密;改性后木屑(MS)表面光滑、结构松散、孔径变大。化学改性除掉了原本粘附在木质素、半纤维以及纤维素非结晶区的一些可提取物和灰分使得改性后木屑表面变光滑。原本致密的结构变得疏松多孔,增大了纤维素的表面积,有利于吸附的进行。

图1 木屑改性前后SEM像
Fig. 1 SEM images of sawdust before and after modification
2.2 结构分析
图2所示为木屑改性前后的XRD谱。由图2可明显看出MS的结晶度降低,可能是由于化学改性改变了木屑纤维素的聚集态结构,且破坏了其有序的结构和结晶区。天然纤维素的衍射图谱中2θ为22°和16°有一个主峰和一个次峰[25-26],RS衍射图谱与其一致。
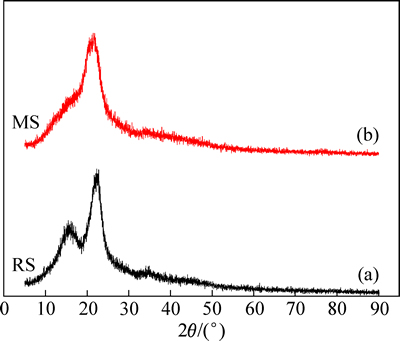
图2 木屑改性前后SRD谱
Fig. 2 XRD patterns of sawdust before (a) and after (b) modification
主峰衍射强度较大,表示高度有序的纤维素结晶区,说明RS中纤维素处于高度有序的聚集状态,羟基之间以强有力的氢键连接。次峰由有序度较低的多糖结构产生。相较于RS,MS在16°的波峰几乎消失,22°的波峰变化很小,说明接枝共聚反应对多糖结构破坏严重,纤维素结晶破坏则较难,这是因为多糖结构结晶层间距较大,容易受到改性试剂的渗透侵蚀。但纤维素结晶面的出峰位置向小角度偏移,说明该晶面间距在增加,这可能是因为改性试剂渗透到纤维素结晶结构内部,形成了柱撑作用。
2.3 单因素实验结果
2.3.1 环氧氯丙烷添加量对改性木屑吸附U(VI)效果的影响
图3所示为环氧氯丙烷(ECH) 添加量对吸附效果的影响。由图3可知,随着ECH添加量的增加,吸附率先增加后降低,ECH添加量为8 mL时,吸附率最大,为96.04%。ECH分子具有较高的化学活性,极易形成正碳离子并使三元环开环,当ECH不足时,季铵化反应缺少引入季铵的“桥”,因此影响了季铵的接入量,进而影响吸附效率。随着ECH添加量的增加,这种“桥”越多,接入吸附剂表面的季铵也越多,吸附位点增多,因此吸附率增大。但当木屑纤维素环氧化充分后,过量的ECH自身会发生与醚化反应竞争的水解、开环及自聚等副反应[27-28],从而降低了吸附率。
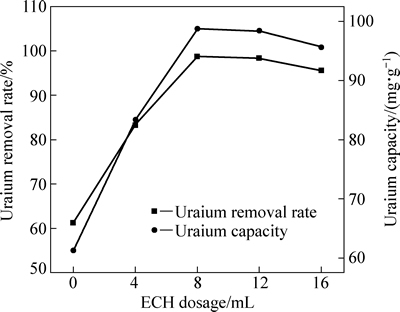
图3 ECH添加量对改性木屑吸附U(VI)效果的影响
Fig. 3 Influence of ECH dosage on adsorption of U(VI) onto modified sawdust
2.3.2 二乙烯三胺添加量对改性木屑吸附U(VI)效果的影响
图4所示为二乙烯三胺(DETA)添加量对吸附效果的影响。随着DETA添加量的增加,U(VI)吸附率先增加后降低,DETA添加量为4 mL时,U(VI)吸附率最大,为98.40%。一方面,较少的交联试剂添加量,制约了ECH与TEA作用形成ECH-季铵盐的聚合物;另一方面,由于DETA量少,致使接入的氨基数量减少,接枝共聚反应产生的季铵基团也相应减少,因此吸附率不高。说明DETA能有效增加木屑表面负载季铵官能团,进而提高吸附性能。当DETA量过高时,环氧化纤维的活性位点不足,并且过多的DETA也会发生一系列副反应,减少了可以负载的活性季铵官能团[29]。
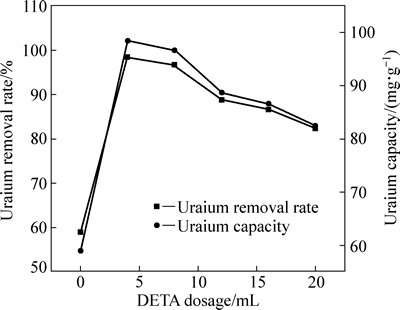
图4 DETA添加量对改性木屑吸附U(VI)效果的影响
Fig. 4 Influence of DETA dosage on adsorption of U(VI) onto modified sawdust
2.3.3 醚化反应时间对改性木屑吸附U(VI)效果的影响
图5所示为醚化反应时间对改性木屑吸附U(VI)效果的影响。由图5可知,随着醚化反应时间的延长,U(VI)吸附率先增大后减小,醚化反应时间为1 h时,U(VI)吸附率最大,为98.40%。反应时间增加的过程中,木屑环氧化变充分,利于木屑的改性。而过长的醚化反应时间容易使ECH发生水解、开环、自聚等副反应,使接枝到纤维素骨架上的环氧基减少,不利于后续交联及接枝反应的进行[27-28]。
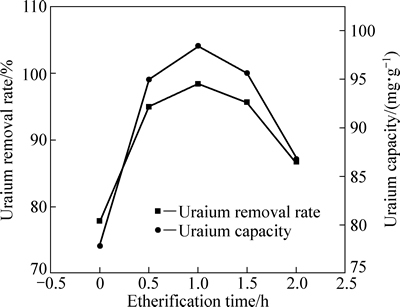
图5 醚化反应时间对改性木屑吸附U(VI)效果的影响
Fig. 5 Influence of etherification time on adsorption of U(VI) onto modified sawdust
2.3.4 接枝反应时间对改性木屑吸附U(VI)效果的影响
图6所示为接枝反应时间对改性木屑吸附U(VI)效果的影响。由图6可知,随着接枝反应时间的延长,改性木屑对U(VI)的吸附率先增大后基本保持不变,最大吸附率为98.40%,此时接枝反应时间为3 h,说明增加接枝反应时间有利于改性木屑接枝季铵官能团。当反应时间短时,TEA的浓度大,纤维上接枝活性点也多,TEA很容易渗入纤维内部,使得接枝率增大。季铵化反应为亲核反应,存在空间位阻效应,随着反应时间的延长,ECH在反应体系舒展完全,故接枝率基本保持不变,吸附率也不再改变。
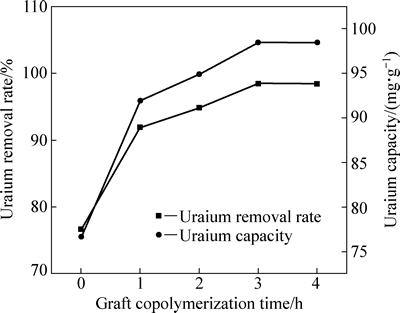
图6 接枝反应时间对改性木屑吸附U(VI)效果的影响
Fig. 6 Influence of graft copolymerization time on adsorption of U(VI) onto modified sawdust
2.4 正交试验结果
根据单因素试验结果,采用正交试验来优化试验结果,研究ECH添加量、DETA添加量、醚化反应时间、接枝反应时间4个因素对10 mg/L U(VI)的吸附率的影响,从而确定最优的制备工艺条件,正交试验结果如表1所列。
由表1中K1~K4及R值可知,对U(VI)吸附率而言,各因素对U(VI)吸附率影响相差不大,其中醚化反应时间影响最大,接枝反应时间影响次之,ECH、DETA添加量影响最小。最优制备工艺条件为:ECH添加量10 mL,DETA添加量为6 mL,醚化反应时间为1 h,接枝反应时间为4 h。
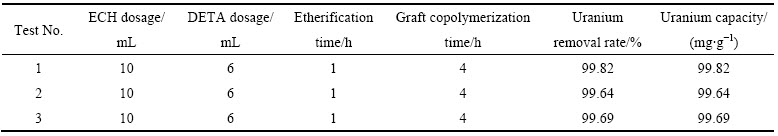
因为正交优化表中没有最优制备工艺条件,所以补做了三组最优工艺条件下的吸附试验,实验结果见表2。从表2可知,在最优工艺条件下制备的季铵盐型螯合吸附剂对U(VI) 的吸附率(平均值)为99.72%,吸附量为99.72 mg/g。
表1 不同因素对U(VI)吸附率影响的正交试验结果
Table 1 Orthogonal test results of effects of different factors on uranium removal rate
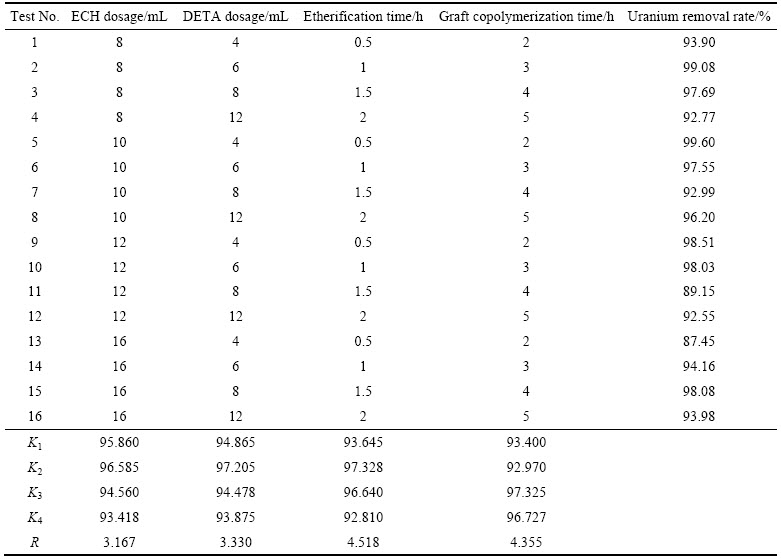
表2 不同因素对U(VI)吸附率影响的补充试验结果
Table 2 Supplement test results of effects of different factors on uranium removal rate
2.5 合成机理分析
图7所示为木屑改性前后的红外图谱。在RS中,3403 cm-1处强且宽的吸收峰为—OH和—NH的伸缩振动。2905 cm-1处的吸收峰为C—H键的伸缩振动,这些C—H键主要来自甲基(—CH3)或亚甲基(—CH2)。木屑吸附水分时造成1647 cm-1处水骨架的弯曲振动。1376 cm-1处为C—H的弯曲振动。897 cm-1处为木屑纤维二糖中的β(1,4)糖苷键振动。天然纤维素的常见吸收峰在3433 cm-1、2917 cm-1、1379 cm-1、1640 cm-1、899 cm-1附近[30]。在RS和MS的红外光谱中上述几个波数附近都有吸收峰且改性前后木屑的红外光谱总体趋势相同,因此可以说化学改性并没有改变木屑的基本结构。
与RS相比,MS光谱中RS在1246 cm-1处的较大峰变成1229 cm-1和1263 cm-1处的两个小峰。C—N官能团振动引起1229 cm-1处的小吸收峰,说明改性后木屑纤维素表面可能成功接入氨基。环氧基的特征峰在1263 cm-1处,说明化学改性过程中发生醚化反应接入环氧基。MS光谱上1736 cm-1处的吸收峰消失,此处峰为木质素中芳香基架振动[31],说明木质素也得到改性。在3430 cm-1处发生了分子间—OH的缔合,说明发生了交联反应。从振动强度上来说,改性后1050 cm-1处N—H官能团的伸缩振动强度较未改性之前明显变大,说明接枝引入更多氨基。
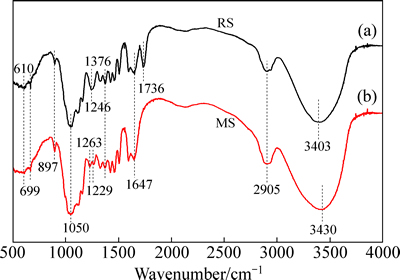
图7 木屑改性前后的红外光谱
Fig. 7 IR spectra of sawdust before (a) and after (b) modification
参与改性反应的主要是木屑纤维中的—OH官能团,ECH的电子云密集在氧原子上,非常活泼,在碱性条件下很容易开环,同时木屑纤维中的—OH与开环后的ECH发生醚化反应,生成3-氯-2-羟丙基纤维素醚;进一步与DETA发生交联反应生成纤维素氨基衍生物。ECH过量时,纤维素氨基衍生物成功引入TEA中的叔胺基团,进而转化为季铵盐。同时DETA中的其他氨基和亚氨基连接到TEA上,发生接枝共聚反应,形成季铵盐,即合成了季铵盐型螯合吸附剂(MS)。季铵盐型螯合吸附剂的制备过程如式 (3)所示:
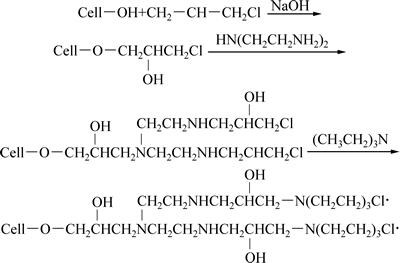 (3)
(3)
3 结论
1) 单因素试验表明:随着ECH、DETA添加量的增加及醚化时间的延长,MS对U(VI)的吸附率先增大后减小,而随着接枝时间的增加,MS对U(VI)的吸附率先增大后基本保持不变。
2) 正交试验表明:最佳制备条件为ECH添加量10 mL、DETA添加量6 mL、醚化反应时间1 h、接枝反应时间4 h,该条件下对U(VI)的吸附率为99.72%,吸附量为99.72 mg/g。各因素对U(VI)吸附率影响相差不大,其中醚化时间影响最大,接枝时间影响次之,ECH及DETA添加量影响最小。
REFERENCES
[1] 潘英杰, 薛建新, 陈仲秋. 我国铀矿冶废物的利用与有用资源的回收[J]. 铀矿冶, 2012, 31(1): 40-45.
PAN Ying-jie, XUE Jian-xin, CHEN Zhong-qiu. Recycling of wastes from uranium mining and metallurgy and recovery of useful resources in China[J]. 2012, 31(1): 40-45.
[2] ZHOU L M, HUANG Z W, LUO T A, JIA Y Y, LIU Z R, ADESINA A A. Biosorption of uranium(VI) from aqueous solution using phosphate-modified pine wood sawdust[J]. J Radioanal Nucl Chem, 2015, 303: 1917-1925.
[3] SAINI A S, MELO J S. Biosorption of uranium by melanin: Kinetic, equilibrium and thermodynamic studies[J]. Bioresour Technol, 2013, 149: 155-162.
[4] LIU M, DONG F, YAN X, ZENG W, HOU L, PANG X. Bio-sorption of uranium by Saccharomyces cerevisiae and surface interactions under culture conditions[J]. Bioresour Technol, 101: 8573-8580.
[5] 周书葵, 曾光明, 刘迎九, 杨金辉, 蒋海燕. 改性羧甲基纤维素对铀吸附机理试验研究[J]. 中国环境科学, 2011, 31(9): 1466-1471.
ZHOU Shu-kui, ZENG Guang-ming, LIU Ying-jiu, YANG Jin-hui, JIANG Hai-yan. Equilibrium, kinetic and thermodynamic study of adsorption of uranium(VI) onto modified CMC[J]. China Environmental Science, 2011, 31(9): 1466-1471.
[6] 彭国文, 丁德馨, 胡 南, 杨雨山, 王晓亮. 纳米 Fe3O4负载啤酒酵母菌对铀的吸附性能与机理[J]. 中国有色金属学报, 2012, 22(2): 604-610.
PENG Guo-wen, DING De-xin, HU Nan, YANG Yu-shan, WANG Xiao-liang. Adsorption properties and mechanism of saccharomyces cerevisiae loaded by nano-Fe3O4 on uranium[J]. 2012, 22(2): 604-610.
[7] MELLAH A, CHEGROUCHE S, BARKAT M. The precipitation of ammonium uranyl carbonate (AUC): thermodynamic and kinetic investigations[J]. Hydrometallurgy, 2007, 85: 163-171.
[8] ZHOU S K, ZENG G M, LIU Y J. Study on removal of uranium(VI) from acid leaching uranium-containing wastewater by modified CMC polymer[J]. Applied Mechanics and Materials, 2011, 100(1): 919-923.
[9] DUBOIS M A, DOZOL J F, NICOTRA C, SEROSE J, MASSIANI C. Pyrolysis and incineration of cationic and anionic ion-exchange resins—identification of volatile degradation compounds[J]. J Anal Appl Pyrol, 1995, 31: 129-140.
[10] XIA L, HU Y X, ZHANG B H. Kinetics and equilibrium adsorption of copper(II) and nickel(II) ions from aqueous solution using sawdust xanthate modified with ethanediamine[J]. Transactions of Nonferrous Metals Society of China, 2014, 24: 868-875.
[11] SABARUDIN A, OSHIMA M, TAKAYANAGI T, HAKIM L, OSHITA K, GAO Yun-hua, MOTOMIZU S. Functionalization of chitosan with 3,4-dihydroxybenzoic acid for the adsorption/collection of uranium in water samples and its determination by inductively coupled plasma-mass spectrometry[J]. Analytica Chimica Acta, 2007, 581(2): 214-220.
[12] ZHANG H J, LIANG H L, CHEN Q D, SHEN X H. Synthesis of a new ionic imprinted polymer for the extraction of uranium from seawater[J]. J Radioanal Nucl Chem, 2013, 298: 1705-1712.
[13] SOYLAK M, YILMAZ E. Sorbent extraction of 4-(2-thiazolylazo) resorcinol (TAR)-metal chelates on Diaion SP-850 adsorption resin in order to preconcentration/ separation[J]. Journal of Hazardous Materials, 2010, 182(1/2/3): 704-709.
[14] JAMES D, WENKATESWARAN G, RAO T P. Removal of uranium from mining industry feed simulant solutions using trapped amidoxime functionality within a mesoporous imprinted polymer material[J]. Microporous Mesoporous Mater, 2009, 119: 165-170.
[15] 张田林, 王佳佳, 朱彩艳, 马慧敏. 季铵盐型螯合树脂的合成及其对铅离子的吸附研究[J].高分子学报, 2013(9): 1219-1225.
ZHANG Tian-lin, WANG Jia-jia, ZHU Cai-yan, MA Hui-min. Synthesis and adsorption properties for Pb(Ⅱ) for a chelation resin containing quaternary ammonium salt[J]. Acta Polymerica Sinica, 2013(9): 1219-1225.
[16] PADMAVATHI R, MINNOLI M, SANGEETHA D. Removal of heavy metal ions from waste water using anion exchange polymer membranes[J]. Int J Plast Technol, 2014, 18(1): 88-99.
[17] 曹 威, 党 志. 季铵化改性稻草吸附去除水中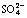 的特性研究[J]. 环境科学学报, 2013, 33(9): 2466-2472.
的特性研究[J]. 环境科学学报, 2013, 33(9): 2466-2472.
CAO Wei, DANG Zhi. Adsorption of sulphate from aqueous solution by quaternary amine-modified rice straw[J]. Acta Scientia Circumstantiae, 2013, 33(9): 2466-2472.
[18] 吴 航, 辛浩波, 杨 锋, 杨金凤, 卢 丹. 螯合纤维对海水中铀的吸脱附性能研究[J]. 合成纤维工业, 2008, 31(6): 7-9.
WU Hang, XIN Hao-bo, YANG Feng, YANG Jin-feng, LU Dan. Adsorption and desorption behavior of chelating fiber for uranium in sea water[J]. China synthetic fiber industry, 2008, 31(6): 7-9.
[19] GB6768—86, 水中微理铀分析方法[S].
GB 6768—86. Methods of analysing microquantity of uranium in water[S].
[20] ZHANG J G. ELDER T J, PU Y Q, RAGAUSKAS A J. Facile synthesis of spherical cellulose nanoparticles[J]. Carbohydrate Polymers, 2007, 69(3): 607-611.
[21] 谢 成, 刘志明, 吴 鹏, 方桂珍, 赵 喣. 碱处理芦苇浆纳米纤维素制备工艺条件优化[J]. 林产化学与工业, 2013, 33: 32-36.
XIE Cheng, LIU Zhi-ming, WU Peng, FANG Gui-zhen, ZHAO Xu. Optimization of preparation technology of alkali pretreated teed pulp nano-cellulose[J]. Chemistry and Industry of Forest Products, 2013, 33: 32-36.
[22] ORLANDO U S, BAES A U, NISHIJIMA W, OKADA M. Preparation of agricultural residue anion exchangers and its nitrate maximum adsorption capacity[J]. Chemosphere, 2002, 48(10): 1041-1046.
[23] ORLANDO U S, OKUDA T, BAES A U, NISHIJIMA W, OKADA M. Chemical properties of anion- exchangers prepared from waste natural materials[J]. React Funct Polym, 2003, 55: 311-318.
[24] 陈华柏, 谢水波, 刘金香, 肖拾红, 曾涛涛, 凌 辉, 王劲松. 厌氧颗粒污泥吸附铀(Ⅵ)的特性与机理[J]. 中国有色金属学报, 2014, 24(9): 2418-2425.
CHEN Hua-bi, XIE Shui-bo, LIU Jin-xiang, XIAO Shi-hong, ZENG Tao-tao, LING Hui, WANG Jin-song. Characteristics and mechanism of uranium (V) absorbed by anaerobic granular sludge[J]. The Chinese Journal of Nonferrous Metals, 2014, 24(9): 2418-2425.
[25] ZHAO H B, KWAK J H, ZHANG Z C, BROWN H M, AREY BW, HOLLADAY J E. Studying cellulose fiber structure by SEM, XRD, NMR and acid hydrolysis[J]. Carbohydrate Polymers, 2007, 68: 235-241.
[26] SEGAL L, CREELY J J, SEGAL L, CREELY J J, JRAEM. An empirical method for estimating the degree of crystallite of native cellulose using the X-ray diffractometer[J]. Textile Research Journal, 1959, 29(10): 786-794.
[27] BAI Y X, LI Y F. Preparation and characterization of crosslinked porous cellulose beads[J]. Carbohydrate Polymers, 2006, 64: 402-407.
[28] 许 醒, 高宝玉, 岳文文, 岳钦艳, 王 宇. 麦草阳离子型吸附剂的合成及对销酸根的去除[J]. 精细化工, 2008, 25(3): 273-276.
XU Xing, GAO Bao-yu, YUE Wen-wen, YUE Qin-yan, WANG Yu. Preparation of anion exchangers from wheat straw and removal of NO3- by modified wheat straw[J]. Fine Chemicals, 2008, 25(3): 273-276.
[29] WANG W Y, YUE Q Y, XU X, GAO B Y, ZHANG J, LI Q, XU J T. Optimized conditions in preparation of giant reed quaternary amino anion exchanger for phosphate removal[J]. Chem Eng J, 2010, 157: 161-167.
[30] LIU C F, XU F, SUN J X, REN J L, CURLING S, SUN R C, FOWLER P, BAIRD M S. Physicochemical characterization of cellulose from perennial ryegrass leaves (Lolium perenne) [J]. Carbohydrate Research, 2006, 341(16): 2677-2687.
[31] PANDEY K K. A study of chemical structure of soft and hardwood and wood polymers by FTIR spectroscopy[J]. Textile Research Journal, 1959, 29: 786-792.
(编辑 王 超)
基金项目:国家自然科学基金资助项目(51174117)
收稿日期:2014-12-29;修订日期:2015-05-27
通信作者:周书葵,教授,博士;电话:13973425348;E-mail:zhoushukui@usc.edu.cn
摘 要:针对铀矿中酸法含铀废水处理的难点,利用木屑制备季铵盐型螯合吸附剂(MS)强化其除铀效果。研究环氧氯丙烷(ECH)添加量、二乙烯三胺(DETA)添加量、醚化反应时间、接枝反应时间对木屑的改性效果及其对废水中U(VI)吸附效果的影响,获取制备MS的最佳工艺条件。对MS进行表征分析并探讨吸附剂的改性机理。单因素实验结果表明:随着ECH、DETA添加量的增加及醚化时间的延长,MS对U(VI)的吸附性能先增大后减小;而随着接枝时间的增加,MS对U(VI)的吸附效果先增大而后基本保持不变。正交优化实验结果表明:对U(VI)吸附性能的影响醚化反应时间最大,接枝反应时间次之,ECH、DETA添加量最小。最优制备条件为ECH添加量10 mL,DETA添加量6 mL,醚化反应时间1 h,接枝反应时间4 h,此时MS对U(VI)的吸附率为99.72%,吸附量达99.72 mg/g。
[1] 潘英杰, 薛建新, 陈仲秋. 我国铀矿冶废物的利用与有用资源的回收[J]. 铀矿冶, 2012, 31(1): 40-45.
[5] 周书葵, 曾光明, 刘迎九, 杨金辉, 蒋海燕. 改性羧甲基纤维素对铀吸附机理试验研究[J]. 中国环境科学, 2011, 31(9): 1466-1471.
[6] 彭国文, 丁德馨, 胡 南, 杨雨山, 王晓亮. 纳米 Fe3O4负载啤酒酵母菌对铀的吸附性能与机理[J]. 中国有色金属学报, 2012, 22(2): 604-610.
[15] 张田林, 王佳佳, 朱彩艳, 马慧敏. 季铵盐型螯合树脂的合成及其对铅离子的吸附研究[J].高分子学报, 2013(9): 1219-1225.
[17] 曹 威, 党 志. 季铵化改性稻草吸附去除水中的特性研究[J]. 环境科学学报, 2013, 33(9): 2466-2472.
[18] 吴 航, 辛浩波, 杨 锋, 杨金凤, 卢 丹. 螯合纤维对海水中铀的吸脱附性能研究[J]. 合成纤维工业, 2008, 31(6): 7-9.
GB 6768—86. Methods of analysing microquantity of uranium in water[S].
[21] 谢 成, 刘志明, 吴 鹏, 方桂珍, 赵 喣. 碱处理芦苇浆纳米纤维素制备工艺条件优化[J]. 林产化学与工业, 2013, 33: 32-36.
[24] 陈华柏, 谢水波, 刘金香, 肖拾红, 曾涛涛, 凌 辉, 王劲松. 厌氧颗粒污泥吸附铀(Ⅵ)的特性与机理[J]. 中国有色金属学报, 2014, 24(9): 2418-2425.
[28] 许 醒, 高宝玉, 岳文文, 岳钦艳, 王 宇. 麦草阳离子型吸附剂的合成及对销酸根的去除[J]. 精细化工, 2008, 25(3): 273-276.


