- Abstract:
- 1 Introduction▲
- 2 Experimental▲
- 3 Results and discussion▲
- 4 Conclusions▲
- References
- Figure
- Fig.1 Colonies on single-layered solid medium
- Fig.2 Crystal violet staining image of Leptospirillum-shaped bacteria
- Fig.3 SEM images of Leptospirillum-shaped bacteria
- Fig.4 TEM image of Leptospirillum-shaped bacteria
- Fig.5 Agarose gel electrophoresis image of 16S rDNA
- Fig.6 Phylogenetic development tree of 16S rDNA
- Fig.7 Growth curves of Leptospirillum ferriphilum strain D1 in media with different initial pH values
- Fig.8 Growth curves of Leptospirillum ferriphilum strain D1 incubated at different temperatures
- Fig.9 Extraction efficiencies of Cu and Fe bioleached by L. ferriphillum strain D1 culture
- Fig.10 Cu and Fe extraction efficiencies bioleached by A. ferrooxidans culture
J. Cent. South Univ. Technol. (2007)04-0467-07
DOI: 10.1007/s11771-007-0091-3![]()
Isolation of Leptospirillum ferriphilum by single-layered solid medium
LIU Jian-she(柳建设)1,2, XIE Xue-hui(谢学辉)1, XIAO Sheng-mu(肖升木)1,
WANG Xiu-mei(王秀美)1, ZHAO Wen-jie(赵文杰)1, TIAN Zhuo-li(田卓力)1
(1. School of Mineral Processing and Bioengineering, Central South University, Changsha 410083, China;
2. College of Environmental Science and Engineering, Donghua University, Shanghai 201620, China)
_____________________________________________________________________
Abstract:
According to physiological and biochemical characteristics of Leptospirillum ferriphilum, a strain of object bacteria was isolated successfully. Bacteria were enriched by selective liquid medium and plated on designed single-layered agar solid medium. Colony was cultured and bacteria were collected. The morphologies of the object bacteria were observed using crystal violet staining, scanning electron microscope(SEM) and transmission electron microscope (TEM). The result of 16S rDNA identification shows that this bacterium belongs to Leptospirillum ferriphilum and it is named as Leptospirillum ferriphilum strain D1. These results indicate that this new single-layered agar solid medium is efficient and simple for isolation of Leptospirillum ferriphilum. Additionally, physiological-biochemical characteristics show that the optimum initial pH value and its growth temperature are 1.68 and 40 ℃, respectively. The culture of it is used to leach a complex concentrate chalcopyrite, the leaching efficiencies of copper and iron are 1.93 % and 13.74 %, respectively, and it is more effective than the A.ferrooxidans culture in the leaching of the complex concentrate chalcopyrite.
Key words:
_____________________________________________________________________
1 Introduction
Members of Leptospirillum genus include Leptospirillum ferrooxidans (group Ⅰ), Leptospirillum ferriphilum (group Ⅱ) and Leptospirillum (group Ⅲ)[1]. They are acidophilic, chemolithoautotrophic, gram-negative bacteria that are morphologically helical curved rods[2], and they have been formally recognized as coherent bacteria[3]. Leptospirillum ferrooxidans and Leptospirillum ferriphilum have been isolated successfully, but there are no reports of Leptospirillum (group Ⅲ) isolated in pure culture[4]. Leptospirillum species are thought to be iron-oxidizing organisms[5]. Although Acidithiobacillus ferrooxidans is the first microbe isolated from an acidic leaching environment and considered to be the primary biological catalyst in biomining processes, Leptospirillum species have been found to be the dominant iron-oxidizing bacteria in industrial continuous-flow biooxidation tanks, such as those used for the treatment of gold-bearing arsenopyrite concentrates[6-8]. In the biooxidation tanks with high concentration ratio of Fe3+ to Fe2+ (c(Fe3+)/c(Fe2+)), the conditions of high temperature (above 40 ℃) and low pH value (below 1.0) are less inhibitory to Leptospirillum species than to A. ferrooxidans[9], which shows that Leptospirillum species may play the most important role in bioleaching process above 40 ℃.
Although Leptospirillum species grow relatively easily in liquid medium, the growth on common solid medium is poor due to the presence of some inhibitors. The gelling agent agar is a polysaccharide and the produced organic compounds of soluble oligosaccharide and monosaccharide under acidic conditions are inhibitory to iron-oxidizers (particularly to the more sensitive Leptospirillum ferrooxidans)[10-11]. To overcome the problems, various alternatives have been suggested, such as silica gel, gelrite and agarose. As the derivative of agar, agarose has been the most favoured alternative gelling agent for solid medium. For an example, the FeTSBo media gelled with agarose support the growth of all strains of A. ferrooxidans and Leptospirillum ferrooxidans tested. But, it is more expensive than agar. A novel approach was described by JOHNSON et al[10, 12]. By incorporating an acidophilic heterotroph into the underlayer of a double-layer medium, the concentration of monosaccharide in gelled medium decreases significantly, which results in a dramatic increase in plating efficiency of most strains of A. ferrooxidans and also the growth of Leptospirillum ferrooxidans that had been reported not to grow on solid media[13]. In China, LIU et al[14-15] in Shandong University firstly used this bilayered agarose solid medium by incorporating heterotroph Acidiphilium SJH into the underlayer, to isolate Leptospirillum ferriphilum strain successfully.
The double-layer agarose medium for isolation Leptospirillum ferriphilum is efficient, but it uses agarose as gelling agent, and especially, needs heterotroph bacteria as assistant bacteria, which makes the isolation procedure more complex. For isolation of Leptospirillum bacteria, a single-layered agar medium was designed, and a strain of Leptospirillum ferriphilum was obtained successfully, named as Leptospirillum ferriphilum strain D1. This new isolation method is more efficient and simple for isolation of Leptospirillum ferriphilum compared to other methods described above.
2 Experimental
2.1 Original bacteria sample and mineral sample
Original bacteria sample was enrichment culture of acid mine drainage (obtained from Zhongtiaoshan Copper Mine, Shanxi Province).
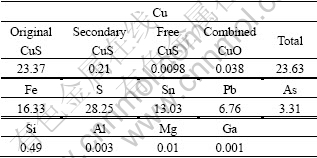
The tested complex concentrate chalcopyrite was obtained from Yaoganxian Copper Mine, Hunan Province. It was fine-milled by a stirred ball to particle size d of 74 μm. The X-ray diffraction results show that it is mainly constituted of stannite (Cu2FeSnS4), chalcopyrite (CuFeS2) and galena (PbS). Table 1 shows the chemical characteristics of this chalcopyrite. It can be seen that the main constituent is original CuS, which is difficult to be leached. In addition, there are high concentrate Sn, Pb and As in the chalcopyrite.
Table 1 Composition of complex concentrate chalcopyrite (mass fraction, %)
2.2 Culture medium
Based on 9K media, the selective liquid medium and solid medium were designed.
The compositions of the liquid medium were as follows: (NH4)2SO4 3 g, KCl 0.1 g, K2HPO4 0.5 g, MgSO4·7H2O 0.5 g, Ca(NO3)2 0.01 g, MnSO4 1.0 g, Al2(SO4)3·18H2O 2.1 g, FeSO4·7H2O 48.0 g, distilled water 1 000 mL and H2SO4 (used to modulate the pH value to 1.65). The liquid medium was sterilized in steam at 121 ℃ for 15 min.
The solid medium was constituted of two parts: Part Ⅰ, liquid medium 600 mL; agar 15 g/L, 200 mL distilled water, which were individually sterilized in steam at 110 ℃ for 30 min; Part Ⅱ, FeSO4·7H2O 48.0 g/L and KSCN 15.0 g/L, 200 mL distilled water, which were aseptic filtrated. Part Ⅰand Part Ⅱwere mixed, and the pH value was adjusted to 3.0 for plate spreading.
2.3 Enrichment and plating
The initial bacteria mixture was cultured in 100 mL selective liquid medium in a 250 mL flask. It was put in a rotating shaker with a speed of 190 r/min at 40 ℃. After 3-4 times of enrichment (10-fold diluted every time), the Leptospirillum-shaped bacteria dominated in the culture which was observed by optical microscope (Olympus CX 31).
1 mL of this culture was diluted for 100-fold with distilled water, 200 μL of which was plated on a disk with single-layered agar solid medium, and incubated at 38.5 ℃ for about 14 d.
2.4 Morphology observation
The colony was cultured using the liquid medium and then the Leptospirillum-shaped bacteria were collected for further study.
The subcellular structure of the Leptospirillum- shaped bacteria was further examined by olympus CX 31 optical microscope, JEOL-5600SL scanning electron microscope (SEM) and JEM1230 transmission electron microscope (TEM), respectively.
2.5 Identification of 16S rDNA
DNA extraction and purification of this bacterium were according to the protocols described by ZHOU et al[16].
16S rDNA genes were amplified by polymerase chain reaction(PCR) in 50 ?L mixtures containing purified DNA 2 ?L (approximately 50 ng/?L), 10× PCR buffer (Perkin Elmer, Norwalk, Conn.) 5 ?L, dNTPs (10 mmol/L) 4 ?L, MgCl2 (25 mmol/L) 3 ?L, forward primer 27F (62.5 μmol/L) 1 ?L and reverse primer 1492R (62.5 μmol/L) 1 ?L, Taq DNA polymerase (1.5 U/?L) (Fermentas Corporation) 1 ?L. In the reaction, the sequences of bacteria-specific 27F and the universal 1492R are 5′-AGAGTTTGATCCTGGCTCAG-3′ and 5′-CGGCTACCTTGTTACGACTT-3′, respectively[17]. A gene amplifier (Biometra, T-Gradient, Genman) was used to incubate reactions through an initial denaturation at 94 ℃ for 2 min, followed by 35 cycles of 94 ℃ for 40 s, 55 ℃ for 30 s, and 72 ℃ for 1 min, and finally at 72 ℃ for 10 min. The PCR products with expected size of about 1.5 kb were pooled and purified using Promega PCR purification columns according to the manufacture’s instructions.
The 16S rDNA sequences were tested by Invitrogen corporation using 3730 automatic sequencing equipment.
Phylogenetic affiliation of the 16S rDNA sequence was initially estimated using the program BLAST—a basic search tool of the National Center for Biotechnology Information (http://www.ncbi. nln.nih. gov/BLAST). Based on the phylogenetic results, appropriate subsets of 16S rDNA sequences were selected and subjected to a final phylogenetic analysis with CLUSTAL V, a phylogenetic development tree was built.
The 16S rDNA sequence had been submitted to GenBank with accession number DQ665909.
2.6 Physiological-biochemical characteristics
Bacteria were cultured in 100 mL selective liquid medium in 250 mL flasks with different initial pH values: 0.45, 1.00, 1.68, 2.00, 2.53, 3.53, 4.37, 5.66. They were put in a rotating shaker with a speed of 190 r/min, at 40 ℃.
Bacteria in selective liquid media were incubated at 30, 40, 45, 50 and 60 ℃, respectively, with the same initial pH value. Flasks were put in a rotating shaker with a speed of 190 r/min.
The concentrations of bacteria during the incubation time were detected by directly counting using optical microscope.
2.7 Leaching experiments
Leaching experiments of the complex concentrate chalcopyrite were carried out in 500 mL flasks with 300 mL 9K medium. The pulp density of concentrate chalcopyrite was 5.0 g/L. The initial pH was 1.68. The inoculums of L. ferriphillum culture and A. ferrooxidans culture were 10% (volume fraction), respectively, and were conducted in duplicated. Sterile controls were also run by replacing the bacterial inoculum by an equal volume of related medium. During the leaching, distilled water was added as compensate for evaporation every day, and the amounts of copper and iron released were determined by atomic absorption spectrophotometry every three days.
3 Results and discussion
3.1 Isolation by plating
After incubated for about 14 d, 10-20 colonies formed on each plate. The image of one plate is shown in Fig.1, where arrows indicate some colonies. The solid medium shows a brownish red color due to the adding of KSCN, while the colonies show a yellow color that are distinguishable. The colonies are round and with smooth surface.
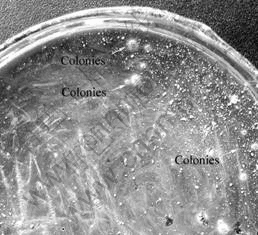
Fig.1 Colonies on single-layered solid medium
Colonies can form on the single-layered agar solid medium successfully mainly due to the presence of KSCN. It has been reported that KSCN can interact with Fe3+ to form complex compounds, which decreases the production of ferroalumen to facilitate the colonies forming[18]. Additionally, JOHNSON et al[12] found that the concentration of monosaccharides and soluble carbohydrates varied with the incubation temperature. And, the pH value has been reported to affect the plating efficiency of solid medium, as the rate of agar hydrolysis will be pH dependent. So, the incubation temperature and pH value selected in this study may help to reduce the negative effect of monosaccharides and improve the efficiency of colonies forming.
3.2 Morphology of bacteria
The morphology of Leptospirillum-shaped bacteria stained by crystal violet was observed using SEM and TEM. The results are shown in Figs.2-4.
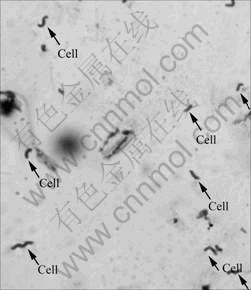
Fig.2 Crystal violet staining image of Leptospirillum-shaped bacteria
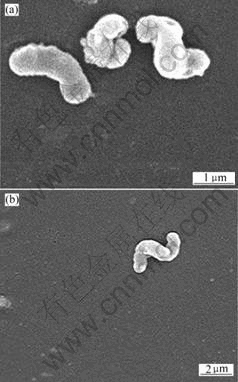
Fig.3 SEM images of Leptospirillum-shaped bacteria
(a) Higher magnification; (b) Lower magnification
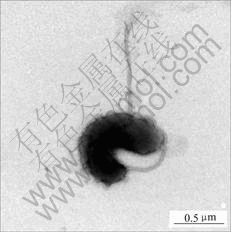
Fig.4 TEM image of Leptospirillum-shaped bacteria
The image in Fig.2 is observed by Olympus CX31 optical microscope under 100× oil immersion les. It can be seen from Figs.2 and 3 that bacteria cells are small curve rod-shaped or helical with size of 0.5 μm×1.5 μm×3.0 μm. These morphology characters are in accordance with the previous reports about Leptospirillum ferriphilu, by SAND et al[19] and NICOLETTE et al[6]. Young cells are vibrio, but after culture more than 4 d, cells are mostly spiral with 2-5 turns. The different shapes of cells in the present study show different developing stages.
It can be seen from that Fig.4, the cells are vibrio-shaped, and have a single polar flagellum. Flagellum is an important morphology character in identification and classification of bacteria. According to the report by SAND et al[19], Leptospirillum ferriphilum cells are gram negative, spore forming, and motile by means of a single polar flagellum. The result in Fig.4 shows that this Leptospirillum-shaped bacterium is similar to Leptospirillum ferriphilum.
3.3 Phylogenetic analysis if 16S rDNA
The 16S rDNA of Leptospirillum-shaped bacterium was amplified with primers 1492R and 27F. The PCR amplification product was detected by 0.8% agarose gel electrophoresis with ultraviolet(UV). The result is shown in Fig.5. The length of object fragment is about 1 500 bp.
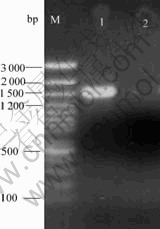
Fig.5 Agarose gel electrophoresis image of 16S rDNA
M— DNA marker; 1—16S rDNA from Leptospirillum-shaped bacteria; 2—Blank control
The object fragment was sequenced. The accurate length is 1 492 bp. It was submitted to the GenBank and obtained the accession number DQ665909. Based on the homology of 16S rDNA, the phylogenetic development tree is built and shown in Fig.6.
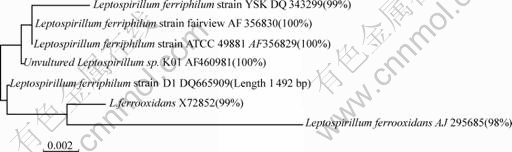
Fig.6 Phylogenetic development tree of 16S rDNA
The Leptospirillum-shaped bacteria’s 16S rDNA sequence has 100% similarity to Leptospirillum ferriphilum sp. K01, Leptospirillum ferriphilum strain ATCC 49881 and Leptospirillum ferriphilum strain Fairview, 99 % similarity to L. ferrooxidans X72852 and 98% to Leptospirillum ferrooxidans AJ295685. Thisresult shows that this strain belongs to the Leptospirillum ferriphilum. And it is named as Leptospirillum ferriphilum strain D1 (shown in Fig.6).
3.4 Physiological-biochemical characteristics
3.4.1 Optimal initial pH value for growth
The growth situation of Leptospirillum ferriphilum strain D1, indicated by the concentration of bacteria, in 8 liquid media with different initial pH values is shown in Fig.7.
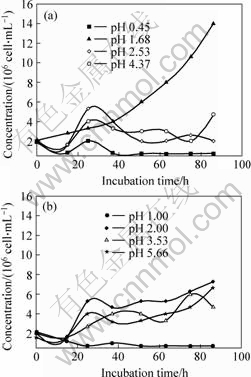
Fig.7 Growth curves of Leptospirillum ferriphilum strain D1 in media with different initial pH values
(a) pH values are: 0.45, 1.68, 2.53, 4.37; (b) pH values are: 1.00, 2.00, 3.53, 5.66
Based on these data in Fig.7, the pH 1.68 is the optimum condition for growth of Leptospirillum ferriphilum in this study.
3.4.2 Optimal temperature for growth
Bacteria in liquid media were incubated under different temperatures with the initial pH value of 1.68. The growth curves of bacteria are shown in Fig.8.
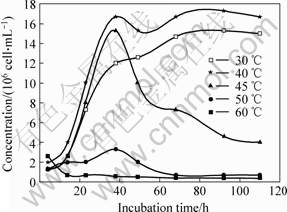
Fig.8 Growth curves of Leptospirillum ferriphilum strain D1 incubated at different temperatures
According to the plot, bacteria incubated at 40 ℃ grow best and have the highest concentration. At 30 ℃, the bacteria grow well too, at the temperature higher than 50 ℃, the growth is inhibited, and 60 ℃ is lethal to its growth. Therefore, the optimum temperature for culturing Leptospirillum ferriphilum is 40 ℃ in this study.
3.5 Leaching results of copper and iron
3.5.1 Leaching by L. ferriphillum strain D1 culture
The copper and iron extraction efficiencies of this complex concentrate chalcopyrite leached by L. ferriphillum strain D1 culture are shown in Fig.9.
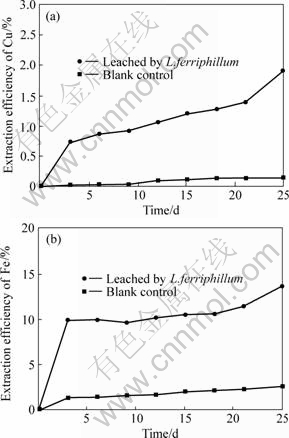
Fig.9 Extraction efficiencies of Cu and Fe bioleached by L. ferriphillum strain D1 culture
(a) Cu; (b) Fe
With the increase of the bioleaching time, the extraction efficiencies of copper and iron increase. After running for 25 d, the extraction efficiencies reach the maximum: 1.93 % for copper and 13.74 % for iron.
3.5.2 Leaching by A. ferrooxidans culture
The copper and iron extraction efficiencies leached by A. ferrooxidans culture are shown in Fig.10.
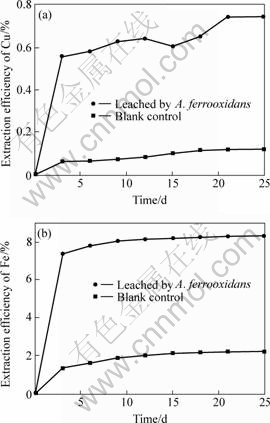
Fig.10 Cu and Fe extraction efficiencies bioleached by A. ferrooxidans culture
(a) Cu; (b) Fe
With the increase of the bioleaching time, the extraction efficiencies of copper and iron increase. After running for 25 d, the extraction efficiencies reach the maximum: 0.74 % for copper and 8.35 % for iron.
Compared to the related blank control experiment, the extraction efficiency of iron of this complex concentrate chalcopyrite by two bacterial cultures is always higher than that of copper. These results indicate that bacteria play a very important role in the leaching process, and the leaching of iron is easier than that of copper. Though the final extraction efficiencies of copper and iron are low, the results still indicate that the L. ferriphillum strain D1 culture is more effective than the A.ferrooxidans culture in leaching of the complex concentrate chalcopyrite in this study. Certainly, the low leaching efficiency may due to many factors, such as the mineralogical characteristics of the sulphide, the particle size, the pulp density, the filled volume, inoculum of bacteria, the incubation time. This concentrate chalcopyrite has high contents of Sn, Pb and As, which may be toxic to bacteria, and bacteria in this bioleaching system have to adapt to the environment firstly, it is a time called lag phase. All of these may influence the extraction of copper and iron by bacteria.
4 Conclusions
1) Leptospirillum ferriphilum strain D1 is isolated by a new single-layered agar solid medium successfully. This medium is more efficient and simpler for isolation of Leptospirillum ferriphilum compared to other methods. In this medium, it is presumed that KSCN facilitates the forming of Leptospirillum ferriphilum colonies. And, the incubation temperature and pH value selected here also are helpful.
2) The cells of Leptospirillum ferriphilum strain D1 observed by optical microscope, SEM and TEM are small curve rod-shaped or helical in different developing stages, with size of 30 μm×1.5 μm×0.5 μm. The bacterium moves by means of a single polar flagellum. According to the molecular method 16S rDNA identification, the bacterium exhibits 100% similarity to Leptospirillum ferriphilum sp. K01, Leptospirillum ferriphilum strain ATCC 49881 and Leptospirillum ferriphilum strain Fairview, 99% similarity to L. ferrooxidans X72852 and 98% similarity to Leptospirillum ferrooxidans AJ295685.
3) The result of physiological-biochemical characteristics’ research shows that the optimum initial pH value is 1.68 and the optimal temperature is 40 ℃ for growth.
4) The leaching efficiencies of copper and iron by L. ferriphilum are 1.93 % and 13.74 %, respectively, and by A. ferrooxidans are 0.74 % and 8.35 %, respectively. The results reveal that the complex concentrate chalcopyrite is hard to be leached by bacteria; there are many factors to influence the extraction efficiency. And the L. ferriphilum culture is more effective than the A. ferrooxidans culture in this leaching study.
References[1] BAKER B J, BANFIELD J F. Microbial communities in acid mine drainage[J]. FEMS Microbiology Ecology, 2003, 44(2): 139-152.
[2] PIVOVAROVA T A, MARKOSYAN G E, KARAVAIKO G I. The auxotrophic growth of Leptospirillum ferrooxidans[J]. Microbiology, 1981, 50(1): 339-344.
[3] HIPPE H. Leptospirillum gen. nov. (ex Markosyan 1972), nom. rev., including Leptospirillum ferrooxidans sp. nov. (ex Markosyan 1972), nom. rev. and Leptospirillum thermoferrooxidans sp. nov. (Golovacheva et al. 1992) [J]. Int J Syst Evol Microbiol, 2000, 50(5): 501-503.
[4] DRUSCHEL G K, BAKER B J, GIHRING T, et al. Acid mine drainage biogeochemistry at Iron Mountain California [J]. Eochem Trans, 2004, 5(2): 13-17.
[5] RAWLINGS D E, SILVER S. Mining with microbes[J]. Bio/Technology, 1995, 13(2): 773-778.
[6] NICOLETTE J C, RAWLINGS D E. Molecular relationship between two groups of the genus Leptospirillum and the finding that Leptospirillum ferriphilum sp. nov. dominates south african commercial biooxidation tanks that operate at 40 ℃[J]. Appl Environ Microbiol, 2002, 68(1): 838–845.
[7] RAWLINGS D E. Restriction enzyme analysis of 16S rRNA genes for the rapid identification of Thiobacillus ferrooxidans, Thiobacillus thiooxidans and Leptospirillum ferrooxidans strains in leaching environments[C]// JEREZ C A, VARGAS T, TOLEDO H, et al. Biohydrometallurgical Processing, vol. II. Santiago: University of Chile Press, 1995: 9–17
[8] RAWLINGS D E, CORAM N J, GARDNER M N, et al. Thiobacillus caldus and Leptospirillum ferrooxidans are widely distributed in continuous flow biooxidation tanks used to treat a variety of metal containing ores and concentrates[C]// AMILS R, BALLESTER A. Biohydrometallurgy and the Environment: toward the Mining of the 21st Century, Part A. Amsterdam: Elsevier, 1999: 777–786.
[9] RAWLINGS D E, TRIBUTSCH H, HANSFORD G S. Reasons why ‘Leptospirillum’-like species rather than Thiobacillus ferrooxidans are the dominant iron-oxidizing bacteria in many commercial processes for biooxidation of pyrite and related ores[J]. Microbiology, 1999, 145(3): 5–13.
[10] JOHNSON D B. Selective solid media for isolating and enumerating acidophilic bacteria[J]. Journal of Microbiological Methods, 1995, 23(2): 205-218.
[11] TUOVINEN O H, KELLY D P. Studies on the growth of Thiobacillus ferrooxidans use of membrane filters and ferrous iron agar to determine viable numbers and comparison with 14CO2- fixation and iron-oxidation as measures of growth[J]. Arch Mikro- boil, 1973, 88(10): 285-298.
[12] JOHNSON D B, MCGINNESS S. A highly efficient and universal solid medium for growing mesophilic and moderately thermophilic, iron-oxidizing, acidophilic bacteria[J]. Microbiol Methods, 1991, 13(7): 113-122.
[13] HARRISON J. A P. The acidophilic thiobacilli and other acidophilic bacteria that share their habitat[J]. Ann Rev Microbial, 1984, 38(2): 265-292.
[14] LIU Ying, LIU Xiang-mei, TIAN Ke-li. Growth and morphology of Leptospirillum ferrooxidans on solid medium[J]. Microbiology, 2003, 30 (6): 70-72. (in Chinese)
[15] LIU Ying, QI Fang-jun, LIU Xiang-mei. Phylogenetic analysis for 16S rDNA sequence of the vibrio shaped chemoautolithotrophic iron-oxidizing bacterium ML-04[J]. Journal of Shandong University, 2004, 39 (5): 112-115. (in Chinese)
[16] ZHOU J, BRUNS M A, TIEDJE J M. DNA recovery from soils of diverse composition[J]. Appl Environ Microbiol, 1996, 62(2): 316–322.
[17] LANE D J. 16S/23S rRNA sequencing[C]// STACKEBRANDT E, GOODFELLOW M. Nucleic Acid Techniques in Bacterial Systematics. Chichester: John Wiley & Sons, 1991: 115–175.
[18] ZHANG Zai-hai, QIU Guan-zhou, HU Yue-hua, et al. The investigation of the colony isolation of thiobacillus ferrooxidans[J]. Multipurpose Utilization of Mineral Resources, 2001, (1): 19-23. (in Chinese)
[19] SAND W, ROHDE K, SOBOTKE B, et al. Evaluation of Leptospirillum ferrooxidans for leaching[J]. Appl Environ Microbiol, 1992, 58(1): 85–92.
_____________________
Foundation item: Projects(50374075, 50321402) supported by the National Natural Science Foundation of China; Project(2004CB619204) supported by the National Basic Research and Development Program of China; Project(200549) supported by the Specialized Research Fund of the National; Excellent PhD Thesis
Received date: 2006-01-11; Accepted date: 2006-09-27
Corresponding author: LIU Jian-she, Professor; Tel: +86-21-67792523; E-mail: ljscsu@263.net
- Isolation of Leptospirillum ferriphilum by single-layered solid medium


