Trans. Nonferrous Met. Soc. China 28(2018) 77-87
A facile method for fabrication of nano-structured Ni-Al2O3 graded coatings: Structural characterization
Arash YAZDANI1,2, Taghi ISFAHANI3
1. Materials Science and Engineering Program, University of California, San Diego, 9500 Gilman Dr., La Jolla, CA 92093-0418, USA;
2. Department of Mechanical and Aerospace Engineering, University of California, San Diego, La Jolla, CA 92093-0411, USA;
3. Metallurgy & Materials Engineering Department, Golpayegan University of Technology, P. O. Box 87717-65651, Golpayegan, Iran
Received 13 December 2016; accepted 30 June 2017
Abstract:
Powder charges of micron-size Ni and Al2O3 were utilized to deposit nano-structured Ni-Al2O3 composite coatings on an aluminum plate fixed at the top end of a milling vial using a planetary ball mill. Composite coatings were fabricated using powder mixtures with a wide range of Ni/Al2O3 mass ratio varying from 1:1 to plain Ni. XRD, SEM and TEM techniques were employed to study the structural characteristics of the coatings. It was found that the composition of the starting mixture strongly affects the Al2O3 content and the microstructure of the final coating. Mixtures containing higher contents of Al2O3 yield higher volume fractions of the Al2O3 particles in the coating. Though Ni-Al2O3 composite coatings with about 50% of Al2O3 particles were successfully deposited, well-compacted and free of cracks and/or voids coatings included less than 20% (volume fraction) of Al2O3 particles which were deposited from powder mixtures with Ni/Al2O3 mass ratios of 4:1 or higher. Moreover, mechanical and metallurgical bondings are the main mechanisms of the adhesion of the coating to the Al substrate. Finally, functionally graded composite coatings with noticeable compaction and integrity were produced by deposition of two separate layers under identical coating conditions.
Key words:
metal matrix composite; Ni-Al2O3 graded coating; structural characterization;
1 Introduction
Metal matrix composite (MMC) coatings reinforced with ceramic particles have received significant attention due to their relatively low cost and easy production by electro co-deposition technique. A large variety of particles, mainly oxides and carbides, with a wide range of particle size have been successfully co-deposited in diverse metal matrixes. MMC coatings with uniform dispersion of ceramic particles, significantly enhance the hardness and wear resistance compared to the metal itself [1,2].
Functionally graded materials (FGM) are newly- developed types of engineering materials whose characteristics such as composition and/or structure gradually alter, leading to changes in their properties. The change in the characteristics could take place gradually or occur in a discontinuous stepwise way. FGMs can reduce the delamination and spalling of the coatings [3-5].
Graded or non-graded Ni-Al2O3 composite coatings have been the topic of many researches. In these composites, nickel is a corrosion-resistant metal and Al2O3 particles with high hardness increase the wear resistance of the composite. Hence, Ni-Al2O3 composite coatings are often used in applications requiring wear and corrosion resistance [6,7]. Ni-Al2O3 coatings are mainly produced by electrodeposition [1,8] or cold spraying methods [9,10]. However, the former is more frequently used to deposit graded coatings [11].
Compared to other methods for the fabrication of MMC coatings such as physical/chemical vapor deposition (PVD/CVD), the electrodeposition method features more homogenous distribution of reinforcements, reduced waste materials and the capability of continuous processing [12]. Nevertheless, the electrochemical route suffers from limitations such as agglomeration, group deposition and the sedimentation of particles (especially at nano size) which make it difficult to produce composite coatings with a uniform dispersion of particles [11,13].
Mechanical coating (MC) technique which is a combination of mechanical alloying (MA) and surface mechanical attrition treatment (SMAT) processes has been widely used to produce different types of coatings. Strong bonding between the coating and substrate at room temperature and atmospheric pressure are the most outstanding merits of the MC method [14,15]. However, limitations such as size and shape of the substrate might limit its industrial applicability.
Planetary ball mills have been vastly employed for the deposition of various metallic [16,17], inter-metallic [18,19], ceramic [20] and composite [21] coatings. Milling balls [22,23], the container sidewall [24,25], small loaded samples in the vial [18,19] and fixed plates at the top lid of the vial [20,26] have been investigated as different target substrates.
According to the literatures, there is no systematic study on the mechanical deposition of Ni-Al2O3 nanocomposite coatings, either graded or non-graded ones, using any type of apparatuses commonly used in MC technique such as a planetary ball mill. Hence, in this research we aim to study the effects of Ni/Al2O3 mass ratio in the initial charge on the microstructural properties of the produced coatings applied on an aluminum substrate fixed at the top end of the milling vial in a planetary ball mill. It is well known that mechanically deposited coatings feature a gradient of structural and mechanical properties through the thickness of the coating. Nevertheless, these gradient features could be intensified by applying two separate layers with different chemical compositions to improve their mechanical and structural characteristics compared to non-graded coatings. Therefore, in this study, the feasibility of the fabrication of the graded composite coatings will also be studied.
2 Experimental
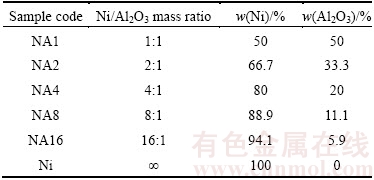
A schematic illustration of the coating set up is shown in Fig. 1. Commercially prepared pure Al plate with a thickness of 3 mm, a microhardness of HV0.05 75 and an average roughness (Ra) of 0.5 μm was fixed at the top end of the milling vial. Before the coating process, the surface of each substrate was cleaned ultrasonically. As-received Ni (Inco, Canada, >99.5%, ~10 μm) and α-Al2O3 powder (USA, >99.5%, ~0.3μm) were used as the coating materials. Powder mixtures with different Ni/Al2O3 mass ratios of 1:1, 2:1, 4:1, 8:1 and 16:1 were prepared. In addition, Ni powder was also tested to produce a plain Ni coating. Details of sample compositions are listed in Table 1.
The deposition process was carried out in a planetary ball mill (P5/4, Fritsch) using a steel jar of 100 mL, operated at a fixed sun-disc rotation speed of 350 r/min for 4 h. In all coating treatments, half of the vial was filled with an equal number of 6- and 8-mm diameter bearing steel balls. The ball-to-powder mass ratio (BPR) used for all coating experiments was 30.

Fig. 1 Schematic presentation of coating setup used in this research
Table 1 Compositions of starting Ni-Al2O3 powder mixtures used in this study
The phase composition of the coatings was studied by X-ray diffraction (XRD) technique using a PANalytical, X’Pert Pro MPD machine with Cu(Kα1:Kα2) radiation (λ=1.5418  ). XRD patterns were labeled according to JCPDS file numbers 04-0787, 04-0850 and 046-1212 for Al, Ni and α-Al2O3 phases, respectively. A qualitative phase analysis of the measured powder diffraction patterns was obtained using the PDF-2 database (ICCD, 2006) in combination with the program MATCH! [27]. For Rietveld refinement, the TOPAS 4-2 program [28] was used. The whole pattern Rietveld refinement was applied to the X-ray diffraction patterns of the obtained coated samples to overcome the problems made from the presence of the several overlapping peaks. Furthermore, to overcome difficulties and limitations of other methods of microstructural analysis and to consider all the benefits of the whole profile fitting methodology, Rietveld’s powder structure refinement procedure based on pseudo-Voigt (pV) profile fitting function was adopted in the present study. The cross-sectional microstructure of the coatings was studied using a VEGA II TESCAN scanning electron microscope (SEM) equipped with energy dispersive X-ray spectroscopy (EDS) system. The volume fraction of the Al2O3 particles in the coatings was calculated from the mass fraction obtained by EDS. Transmission electron microscopy (TEM) observation was also carried out using a LEO 912AB with an operating voltage of 120 kV.
). XRD patterns were labeled according to JCPDS file numbers 04-0787, 04-0850 and 046-1212 for Al, Ni and α-Al2O3 phases, respectively. A qualitative phase analysis of the measured powder diffraction patterns was obtained using the PDF-2 database (ICCD, 2006) in combination with the program MATCH! [27]. For Rietveld refinement, the TOPAS 4-2 program [28] was used. The whole pattern Rietveld refinement was applied to the X-ray diffraction patterns of the obtained coated samples to overcome the problems made from the presence of the several overlapping peaks. Furthermore, to overcome difficulties and limitations of other methods of microstructural analysis and to consider all the benefits of the whole profile fitting methodology, Rietveld’s powder structure refinement procedure based on pseudo-Voigt (pV) profile fitting function was adopted in the present study. The cross-sectional microstructure of the coatings was studied using a VEGA II TESCAN scanning electron microscope (SEM) equipped with energy dispersive X-ray spectroscopy (EDS) system. The volume fraction of the Al2O3 particles in the coatings was calculated from the mass fraction obtained by EDS. Transmission electron microscopy (TEM) observation was also carried out using a LEO 912AB with an operating voltage of 120 kV.
3 Results and discussion
3.1 Visual and SEM observations
After the coating process, photographs of the coated Al substrates were prepared. Typical pictures of some of the coatings are shown in Fig. 2.
It is clearly seen that all coatings have a relatively even appearance. Although, circular rings resulting from the material accumulation, are observed on the surface of some of the coatings (Figs. 2(c) and (d)). These rings are presumably created by the shearing force exerted by the ball collisions on the already-deposited coating layer and consequently the plastic flow of coating material [26]. This phenomenon is common for the coatings produced from the Ni-rich powder mixtures.
Cross-sectional microstructure of the coatings was studied by SEM and the images are shown in Fig. 3. As can be seen from Figs. 3(a) and (b), the composite coatings produced from powder mixtures with Ni/Al2O3 mass ratios of 1:1 and 2:1 have an average thickness of (24±4) μm and (40±7) μm, respectively, which are significantly lower compared to the other coatings with average thicknesses of (85±10) μm. More importantly, some cracks were observed in the coatings deposited from the alumina-rich mixtures (Figs. 3(a) and (b)). These cracks which exist either at the top or bottom layer of the coating could substantially affect the properties of the coating. It seems that there is an optimum value for the Ni/Al2O3 mass ratio of the initial charge above which coatings with acceptable microstructural and mechanical properties are yielded. According to the results obtained in the present research, Ni-Al2O3 composite coatings should be prepared from the mixtures with Ni/Al2O3 mass ratios of 4:1 or greater. No solid conclusion could be made on whether this optimum mass ratio is similar for all metal matrix composites produced by the MC technique. The probable difference between the optimum values might be attributed to different conditions of the coating process and the nature of the reinforcement particles. In the line with this argument, diverse behaviors of various ceramic particles in a similar matrix material have been reported by other researchers [29].
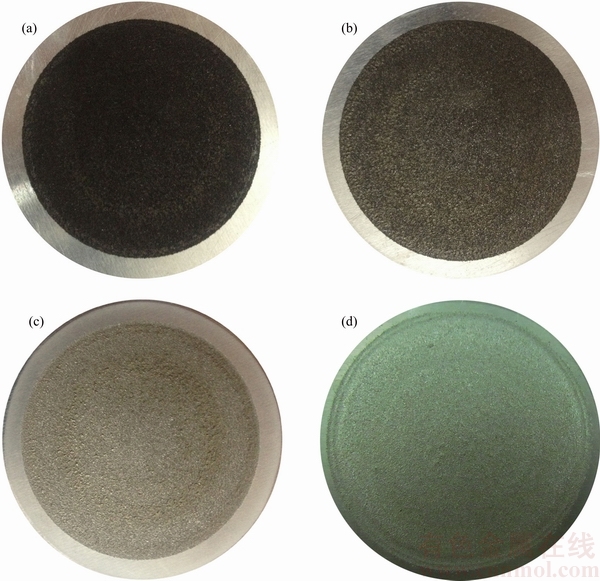
Fig. 2 Photographs of coatings produced from powder mixture containing different Ni/Al2O3 mass ratios of 1:1 (a), 4:1 (b), 16:1 (c) and plain Ni (d) (Diameter of Al substrate is about 50 mm)
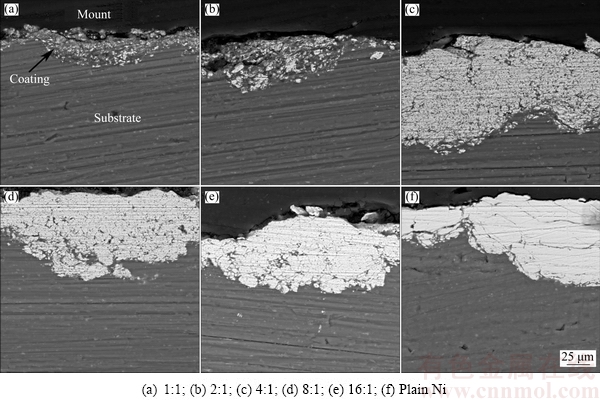
Fig. 3 Cross-sectional SEM images of composite coatings produced from mixtures with different Ni/Al2O3 mass ratios
It is worthwhile mentioning that the ball collisions have induced plastic deformation in the aluminum substrate. As can be seen in Figs. 3(a)-(f), the roughness of the substrate at the coating/substrate interface has significantly increased, leading to the enhancement of mechanical interlocking between the coating and substrate. As proposed by other researchers [15,30], mechanical bonding is almost the main bonding mechanism for mechanically coated soft substrates such as aluminum. Nevertheless, there is an evidence of metallurgical bonding which will be discussed later.
Adequate compaction of the coating and strong bonding between the coating/substrate and ceramic particles/metal matrix are crucial factors affecting the performance of the composite coatings. High- magnification SEM images obtained from the coatings produced from mixtures containing different Ni/Al2O3 mass ratios are shown in Fig. 4. It can be clearly seen that in the case of coatings produced from a charge with a Ni/Al2O3 mass ratio of 2:1 (Fig. 4(a)), the coating layer could be roughly divided to two separate regions:
1) The beneath layer adhered to the substrate with obvious intermixing of metallic Ni and ceramic alumina particles. Strong bonding between the coating and substrate and more importantly the matrix and reinforcement can be concluded.
2) The top layer consisting of Ni and Al2O3 particles with a significantly lower density of Ni particles compared to the beneath one. It is clearly seen that there is a relatively high degree of voids in this layer implying lower adhesive and cohesive strength (lower integrity).
On the other hand, it is well known that ball collisions with already-deposited layer during the coating process, lead to a higher compaction of the outermost layers compared to the innermost ones. This contradiction could be interestingly interpreted by the amount of soft metallic component. As mentioned previously, there is a critical value for the Ni/Al2O3 mass ratio in the initial charge above which coatings with desirable microstructural and mechanical properties could be fabricated. This might be further verified by the cross-sectional image (Figs. 4(b) and (c)). By increasing the Ni content in the starting powder mixture, the integrity of the coating increases remarkably. Figure 4(d) shows the interface between the coating/substrate and Al2O3 particles/Ni matrix. It can be seen that Ni particles are compactly cold-welded, forming a continuous matrix with well surrounded Al2O3 particles. More importantly, there is no crack/void at the interface of the Al2O3 particles/Ni matrix as well as the coating/substrate, indicating an acceptable adhesive and cohesive strength.
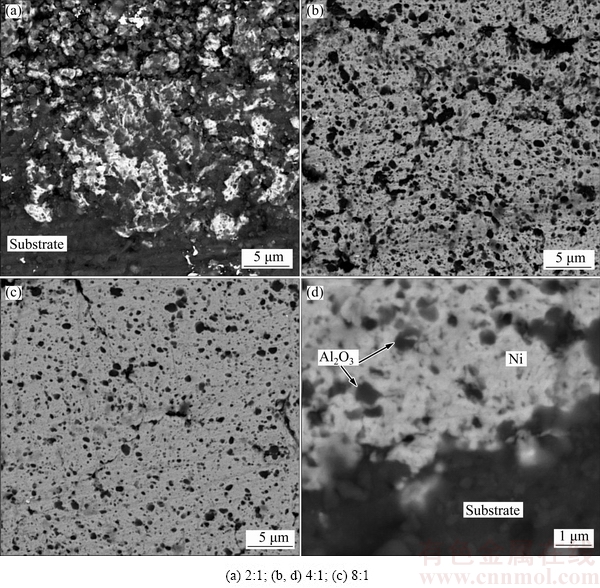
Fig. 4 High magnification cross-sectional SEM images of composite coatings produced from mixtures with different Ni/Al2O3 mass ratios
The distribution of Al2O3 particles in the Ni matrix was examined by elemental distribution map (Fig. 5). Al2O3 particles are uniformly distributed in the Ni matrix anticipating promising functional characteristics. It should be noticed that the homogeneous distribution of reinforcement particles in the matrix is one of the most crucial factors regarding the fabrication of composite coatings. This is a quite challenging issue especially when nano- or submicron-sized ceramic particles are to be incorporated into a metallic matrix by commercialized fabrication methods such as electrodeposition one. Nevertheless, several mechanical and chemical routes have been suggested to prevent the agglomeration or the group deposition of small particles in either bath or coating [31].
In order to determine the amount of incorporated Al2O3 particles in the composite coatings, the cross section of the coatings was examined using EDS technique. The volume fraction of the incorporated Al2O3 particles has been calculated and shown in Fig. 6.
It seems that there is a progressive linear relation between the mass fraction of Al2O3 in the powder mixture and the volume fraction of Al2O3 in the coating. It is worth noticing that composite coatings with high amount of Al2O3 particles (more than 30%, volume fraction) could be produced using mechanical coating technique, although their performance due to the lack of the structural integrity is in doubt.
The masses of the aluminum substrate and steel balls, before and after each coating trail were measured using an electronic balance. As expected, the masses of the substrate and balls increased after each test, but different patterns were observed when comparing all samples. The results are presented in Fig. 7.
It is evident that the rate of the mass gain for either substrate or ball increases as the Ni content of the powder mixture increases. Besides, there is a big jump in the mass gain from Ni/Al2O3 mass ratio of 2:1 to 4:1 which is in good agreement with the increasing coating thickness shown previously (Fig. 3). Both plate-like soft Ni and angular hard Al2O3 particles can strongly adhere to the available surfaces in the grinding media including the milling vial, balls and substrate. Deformed metallic particles with high surface area can adhere strongly to bare or already-coated surfaces. Al2O3 particles, in the form of either sole particles or Ni-Al2O3 composite ones, might also adhere to the grinding media, especially those with lower hardness such as an aluminum substrate. On the other hand, mechano-reactor based vibration chambers and planetary ball mills cannot provide sufficient energy for the dense compaction of ceramic particles [20]. Consequently, in Al2O3-rich coatings there is a high density of cracks and pores in the coating layer or at the coating/substrate interface which facilitates the fracture of the just-deposited layer. That is why the mass gain of the substrate and milling balls in trails using Al2O3-rich powder mixtures (Ni/Al2O3 mass ratios of 1:1 and 2:1) is smaller compared to that of other trails. By increasing the Ni content in the powder mixture (Ni/Al2O3 mass ratio of 4:1 and higher), the amount of the Ni in the coating also increases. Consequently, the integrity (cohesion) of the deposited coating layers on grinding media is enhanced in such a way that they can withstand the energy transferred by the collision of flying balls. Most probably, the kinetic energy of collisions is consumed for the further compaction of already-deposited layers instead of initiation and propagation of cracks and subsequent fracture.
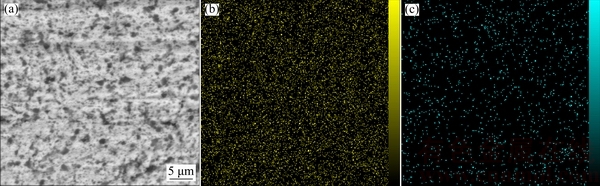
Fig. 5 Cross-sectional SEM image (a) of Ni-Al2O3 composite coating produced from mixture with Ni/Al2O3 mass ratio of 4:1 accompanied by elemental maps of Ni (b) and Al (c)
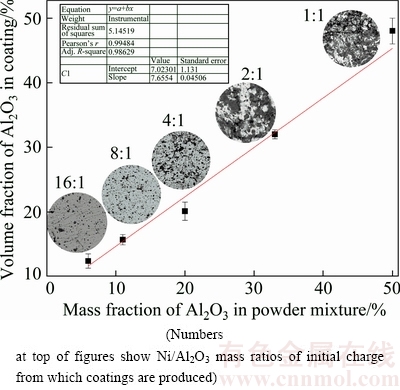
Fig. 6 Relation between amount of Al2O3 in starting powder mixture and incorporated Al2O3 particles in coating

Fig. 7 Variation of mass gain of substrate and balls during coating process for different mass ratios of starting powder mixture
3.2 Phase and structural analysis
The peaks in the XRD patterns of the obtained coatings were revealed and indexed. According to the Rietveld refinement, the broadening of peaks for Ni and Al is related to the reduction in the crystallite size. Furthermore, preferred orientation was observed in produced coatings. Figure 8 shows the X-ray diffraction patterns, the best fit profile obtained by the Rietveld refinement and the difference pattern of the coatings prepared from powder mixtures with Ni/Al2O3 mass ratios of 1:1, 2:1, 4:1, 8:1, 16:1 and plain Ni.
Table 2 shows the results of Rietveld refinements of the XRD patterns corresponding to the produced coatings.
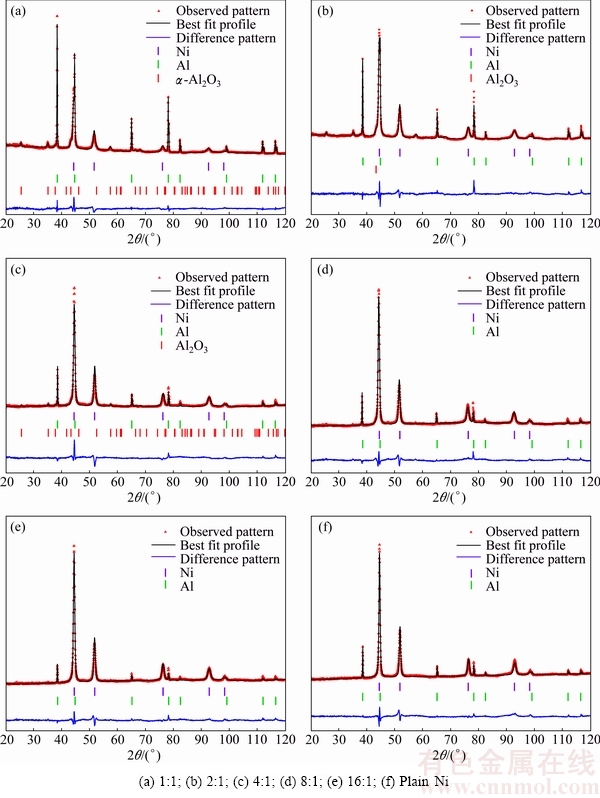
Fig. 8 XRD patterns of coatings produced from different mass ratios of Ni/Al2O3
Due to the small amount of α-Al2O3 present in the coatings produced from mixtures with Ni/Al2O3 mass ratios of 8:1 and 16:1, only Ni and Al are characterized while for other coatings, all three compounds (Ni, Al and Al2O3) are observed. It is worth mentioning that the results of Rietveld refinements for the Al2O3 phase are not shown in Table 2.
According to the Rietveld refinements, by increasing the amount of the Ni powder in the starting mixture, the crystallite size of Ni increases from 87 to 147 nm and the strain decreases from 0.9 to 0.47. Since coating parameters were kept constant for all coating trials, it is expectable that by increasing the amount of the Ni in the starting powder mixture and consequently in produced coatings, a similar amount of kinetic energy is transferred to a greater amount of Ni. Consequently, less plastic deformation would occur, leading to the larger crystallite size and smaller microstrain of the Ni matrix. Similar variation trend is also observed in the crystallite size and microstrain of the Al substrate. Much smaller strain values of Al substrate compared to Ni are because the milling energy is mostly applied to the Ni and Al2O3 powders by direct collision and it has less influence on the Al substrate plate. Moreover, Ni and Al2O3 are in powder state and powders compared to plates can receive and undergo much more strain due to their lower bonding energy, therefore, Al which is in the form of a plate has a higher resistance to deformation. However, based on the results of Rietveld refinement, one may conclude that in all of the coated samples, there is a preferred orientation in Ni and/or the Al substrate (Fig. 9).
Table 2 Results of Rietveld refinements of XRD patterns corresponding to samples prepared from mixtures with Ni/Al2O3 mass ratios of 1:1, 2:1, 4:1, 8:1, 16:1 and plain Ni (D is crystallite size, w is mass fraction, ε is microstrain, a is lattice parameter, Rwp is weighted profile R-factor and GOF is goodness of fit)
According to Fig. 9, the preferred orientation for Ni (Figs. 9(a) and (b)) and Al (Figs. 9(c)-(e)) are shown. As seen in Fig. 9(a), the observed pattern is lower than the calculated best fit profile, indicating that less crystals are in the direction of (002) while in Fig. 9(b), the observed pattern is higher than the calculated best fit profile, indicating preferred orientation in the direction of (111). Therefore, it can be understood that the milling procedure resulted in the preferred orientation from (002) to (111) for Ni. Furthermore, for the Al substrate a similar behavior is seen. As shown in Fig. 9(c), the observed pattern for the (111) is lower than the calculated best fit profile by Rietveld refinement, while for the (311), the calculated best fit profile is lower than the observed pattern (Fig. 9(d)). This shows that the milling procedure results in the preferred orientation of Al from (111) to (311). To obtain high quality and accurate results, the Rietveld refinements were done considering preferred orientation. For example, the (111) peak for Al is shown in Fig. 9(e).
According to Table 2, the lattice parameter of Ni matrix varies between 3.53 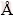 and 3.57
and 3.57 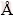 . The change in the lattice parameter of the Ni coating would be an evidence of supporting the formation of Ni(Al) solid solution caused by ball collision and establishment of the metallurgical bonding between Ni matrix and Al substrate. The atomic radii of Ni and Al are 124 and 143 pm, respectively. Lattice parameter of Ni is also 3.524
. The change in the lattice parameter of the Ni coating would be an evidence of supporting the formation of Ni(Al) solid solution caused by ball collision and establishment of the metallurgical bonding between Ni matrix and Al substrate. The atomic radii of Ni and Al are 124 and 143 pm, respectively. Lattice parameter of Ni is also 3.524 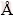 . It is quite expectable that the diffusion of large Al atoms into the Ni lattice leads to the expansion of Ni cell and consequently the increment of Ni lattice parameter. Moreover, it is seen in Table 2 that the higher the amount of the Ni in the coating is, the greater the lattice parameter of Ni is. Presumably, this is due to the diffusion of higher amount of Al into the Ni matrix and expansion of Ni unit cell which leads to the larger Ni lattice parameters. Although the reduction of lattice parameter under exerted compressive force by ball collisions is expected, increase or no change in the lattice parameters has been reported for coatings in which diffusion and solid solution formation occur [20,32].
. It is quite expectable that the diffusion of large Al atoms into the Ni lattice leads to the expansion of Ni cell and consequently the increment of Ni lattice parameter. Moreover, it is seen in Table 2 that the higher the amount of the Ni in the coating is, the greater the lattice parameter of Ni is. Presumably, this is due to the diffusion of higher amount of Al into the Ni matrix and expansion of Ni unit cell which leads to the larger Ni lattice parameters. Although the reduction of lattice parameter under exerted compressive force by ball collisions is expected, increase or no change in the lattice parameters has been reported for coatings in which diffusion and solid solution formation occur [20,32].
3.3 TEM observations
Figure 10 shows the TEM images from the Ni-Al2O3 composite coatings produced from a mixture with a Ni/Al2O3 mass ratio of 4:1. Black arrows in Fig. 10(a), show some of the nanometric Al2O3 particles. Two Al2O3 particles embedded in Ni matrix are presented in Fig. 10(b). As can be seen, even at a high- magnification of TEM image, there is no sign of crack or pore at the interface of the Al2O3 particles and Ni matrix, indicating the strong bonding between the constituents of the coatings. Figure 10(c) presents the cold-welding phenomenon between two large Ni particles. Because of the severe deformation of Ni particles accompanied by the large amount of the energy transferred by the colliding balls, occurrence of the cold welding is quite expectable.
3.4 Synthesis of functionally graded Ni-Al2O3 composite coatings
In order to produce the graded coating, in the first step, a Ni-Al2O3 composite layer was deposited on an Al substrate from a powder mixture with a Ni/Al2O3 mass ratio of 16:1, leading to the inclusion of 12.9% (volume fraction) of Al2O3 particles in the coating. In the second step, the top layer with 21.6% (volume fraction) of Al2O3 particles was deposited from a mixture with a Ni/Al2O3 mass ratio of 4:1. The processing parameters such as BPR, rotation speed and time for each coating stage were set as 30, 350 r/min and 4 h, respectively. Figure 11 shows the cross-sectional SEM images of the coating at low and high magnifications.

Fig. 9 Observed patterns and calculated best fit profiles for Ni (a, b) and Al (c-e) peaks in XRD pattern of coated sample using powder mixture with Ni/Al2O3 mass ratio of 2:1

Fig. 10 TEM images of coating produced from powder mixture with Ni/Al2O3 mass ratio of 4:1
Figure 11(a) clearly shows that the first layer (Ni/Al2O3 mass ratio 16:1), with a high compaction degree, has strongly adhered to the soft Al substrate mainly by mechanical bonding. Moreover, though the coating time for the deposition of both layers is identical, the first layer (thickness (51±8) μm) is significantly thicker than the second one (thickness (14±2) μm). The SEM image with a higher magnification taken from the interface of the two layers (Fig. 11(b)) presents that these layers are well adhered to each other presumably due to the same matrix material of both layers. Furthermore, there are some embedded Al2O3 particles at the interface of the first and second layers which could deteriorate the homogeneity of the interface region and consequently the cohesion strength of the coating.
Figure 12 shows the elemental line analysis of the graded coatings. The concentration of Ni gradually decreases from the first to the second layer while those of Al and O vary with an opposite trend.
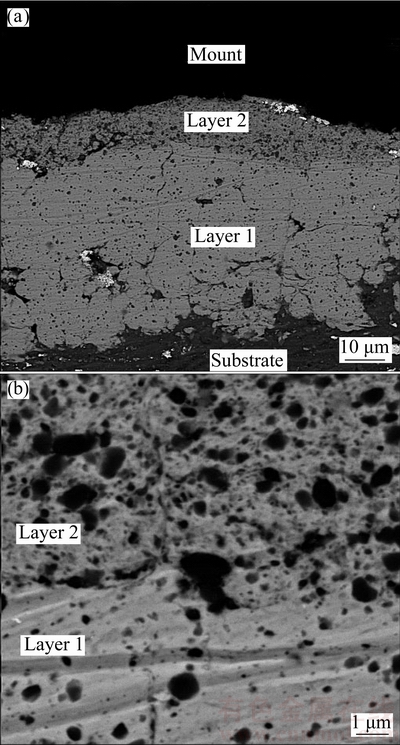
Fig. 11 Cross-sectional SEM images of graded coating at low (a) and high (b) magnifications
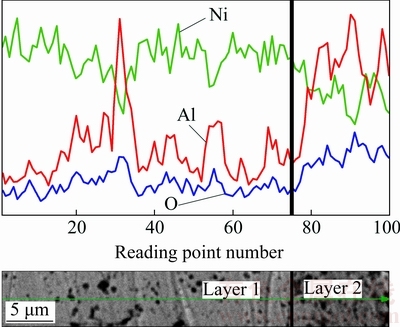
Fig. 12 Line analysis of produced graded coating through coating thickness
4 Conclusions
1) Nano-structured Ni-Al2O3 composite coatings with different amounts of homogeneously incorporated Al2O3 particles were mechanically deposited on the Al substrate fixed at the top end of the vial in a planetary ball mill. It was found that to produce a well compacted Ni-Al2O3 composite coating, there is a critical value of 4:1 for the Ni/Al2O3 mass ratio in the initial charge above which Al2O3 particles are tightly surrounded by the Ni matrix. Though inclusion about 50% (volume fraction) of the Al2O3 particles was achieved in this research, it was found that coatings containing more than 20% (volume fraction) Al2O3 suffer from poor compaction and integrity due to the presence of cracks and pores either through the coating thickness or at interface of the coating/substrate. Moreover, it was found that by increasing the amount of the Ni in the starting powder mixture and consequently in the deposited coating, the crystallite size of the Ni also increased.
2) We also successfully fabricated a graded composite coating consisting of a low-Al2O3 layer beneath and an Al2O3-rich one at the top. Structural analysis revealed that mechanical coating technique (MCT) is capable of fabricating a compact graded coating. Although the MCT suffers from the limitations such as the size and the shape of the substrate to be coated, the results of the current research can shed light on the potential applications of this method for the fabrication of graded coatings.
References
[1] GORAL A, NOWAK M, BERENT K, KANIA B. Influence of current density on microstructure and properties of electrodeposited nickel-alumina composite coatings [J]. Journal of Alloys and Compounds, 2014, 615: s406-s410.
[2] GUL H, KILIC F, ASLAN S, ALP A, AKBULUT H. Characteristics of electro-co-deposited Ni-Al2O3 nano-particle reinforced metal matrix composite (MMC) coatings [J]. Wear, 2009, 267: 976-990.
[3] GARCIA-LECINAA E, GARCIA-URRUTIA I, DIEZ J A, SALVO M, SMEACETTO F, GAUTIER G, SEDDON R, MARTIN R. Electrochemical preparation and characterization of Ni/SiC compositionally graded multilayered coatings [J]. Electrochimica Acta, 2009, 54: 2556-2562.
[4] KIM K S, YOO J H. Formation of bilayer Ni-SiC composite coatings by electrodeposition [J]. Surface and Coating Technology, 1998, 108/109: 564-569.
[5] DONGA Y S, LINA P H, WANG H X. Electroplating preparation of Ni-Al2O3 graded composite coatings using a rotating cathode [J]. Surface and Coating Technology, 2006, 200: 3633-3636.
[6] KUO S L, CHEN Y C, GER M D, HWU W H. Nano-particles dispersion effect on Ni/Al2O3 composite coatings [J]. Materials Chemistry and Physics, 2004, 86: 5-10.
[7] ARUNA S T, EZHIL SELVI V, WILLIAM GRIPS V K, RAJAM K S. Corrosion- and wear-resistant properties of Ni-Al2O3 composite coatings containing various forms of alumina [J]. Journal of Applied Electrochemistry, 2011, 41: 461-468.
[8] GUPTA A, BARKAM S, LAHIRI D, BALASUBRAMANIAM R, BALANI K. Effect of alumina dispersion on microstructural and nanomechanical properties of pulse electrodeposited nickel-alumina composite coatings [J]. Journal of Materials Science and Technology, 2014, 30(8): 808-813.
[9] LI W, HUANG C, YU M, LIU D, FENG Y, LIAO H. Investigation of high temperature oxidation behavior and tribological performance on cold sprayed nickel–alumina composite coating [J]. Surface and Coating Technology, 2014, 239: 95-101.
[10] WINNICKI M, MA LACHOWSKA A, RUTKOWSKA- GORCZYCA M, SOKO-LOWSKI P, AMBROZIAK A, PAWLOWSKI L. Characterization of cermet coatings deposited by low-pressure cold spraying [J]. Surface and Coating Technology, 2015, 268: 108-114.
[11] LAJEVARDI S A, SHAHRABI T, SZPUNAR J A. Synthesis of functionally graded nano Al2O3–Ni composite coating by pulse electrodeposition [J]. Applied Surface Science, 2013, 279: 180–188.
[12] THIEMIG D, BUND A, TALBOT J B. Influence of hydrodynamics and pulse plating parameters on the electrocodeposition of nickel–alumina nanocomposite films [J]. Electrochimica Acta, 2009, 54: 2491-2498.
[13] CORNI I, CHATER R J, BOCCACCINI A R, RYAN M P. Electro co-deposition of Ni-Al2O3 composite coatings [J]. Journal of Materials Science, 2012, 47: 361-373.
[14] REVESZ R, TAKACS L. Alloying and amorphization by surface mechanical treatment [J]. Materials Science Forum, 2010, 659: 239-244.
[15] KOMAROV, S V, ROMANKOV, S E, HAYASHI, N, KASAI E. Nanostructured coatings produced by a novel ultrasonic-assisted method: Coating characterisation and formation mechanism [J]. Surface and Coating Technology, 2010, 204: 2215-2222.
[16] HAO L, LU Y, SATO H, ASANUMA H. Fabrication of zinc coatings on alumina balls from zinc powder by mechanical coating technique and the process analysis [J]. Powder Technology, 2012, 228: 377-384.
[17] POURIAMANESH R, VAHDATI-KHAKI J, MOHAMMADI Q. Coating of Al substrate by metallic Ni through mechanical alloying [J]. Journal of Alloys and Compounds, 2009, 488: 430-436.
[18] ZADOROZHNYY V, KALOSHKIN S, TCHERDYNTSEV V, GOROSHENKOV M, KOMISSAROV A, ZADOROZHNYY M. Formation of intermetallic Ni–Al coatings by mechanical alloying on the different hardness substrates [J]. Journal of Alloys and Compounds, 2014, 586: s373-s376.
[19] CANAKCI A, ERDEMIR F, VAROL T, OZKAYA S. Formation of Fe-Al intermetallic coating on low-carbon steel by a novel mechanical alloying technique [J]. Powder Technology, 2013, 247: 24-29.
[20] YAZDANI A, ZAKERI A. An insight into formation of nanostructured coatings on metallic substrates by planetary ball milling [J]. Powder Technology, 2015, 278: 196-203.
[21] CHEN C, DUAN C, LI Y, FENG X, SHEN Y. Effects of Cu content on the microstructures and properties of Cr-Cu composite coatings fabricated via mechanical alloying method [J]. Powder Technology, 2015, 277: 36-46.
[22] KOBAYASH K. Formation of coating film on milling balls for mechanical alloying [J]. Materials Transaction, JIM, 1995, 36: 134-137.
[23] LU Y, KOBAYASH K, GUAN S, HAO L, YOSHIDA H, ASANUMA H, CHEN J. Influence of oxidation process on photocatalytic activity of photocatalyst coatings by mechanical coating technique [J]. Materials Science in Semiconductor Processing, 2015, 30: 128-134.
[24] LI B, DING R, SHEN Y, HU Y, GUO Y. Preparation of Ti-Cr and Ti-Cu flame-retardant coatings on Ti-6Al-4V using a high-energy mechanical alloying method: A preliminary research [J]. Materials and Design, 2012, 35: 25-36.
[25] GU D, SHEN Y. Microstructures and properties of high Cr content coatings on inner surfaces of carbon steel tubular components prepare d by a novel mechanical alloying method [J]. Applied Surface Science, 2009, 256: 223-230.
[26] YAZDANI A, ZAKERI A. Feasibility of mechanical coating of Al substrate with metallic and non-metallic powders [J]. Iranian Journal of Materials Science and Engineering, 2015, 12: 26-33.
[27] BRANDENBURG K, PUTZ H. MATCH! Phase identification from powder diffraction [Z]. 4th ed. Bonn, 2006.
[28] BRUKER. Topas [M]. 4 ed. Karlsruhe, Germany: Bruker AXS, 2007.
[29] SRIVASTAVA M, GRIPS V K W, RAJAM K S. Influence of SiC, Si3N4 and Al2O3 particles on the structure and properties of electrodeposited Ni [J]. Materials Letters, 2008, 62: 3487-3489.
[30] ROMANKOV S, HAYASAK Y, KALIKOVA G, KOMAROV S V, HAYASHI N, KASAI E. TEM study of TiN coatings fabricated by mechanical milling using vibration technique [J]. Surface and Coating Technology, 2009, 203: 1879-1884.
[31] SABRI M, SARABI A A, NASERI KONDELO S M. The effect of sodium dodecyl sulfate surfactant on the electrodeposition of Ni-alumina composite coatings [J]. Materials Chemistry and Physics, 2012, 136: 566-569.
[32] ROMANKOV S, HAYASAKA Y, HAYASHI N, KASAI E, KOMAROV S V. Ball impact cladding of metals with dissimilar metallic foils [J]. Surface and Coating Technology, 2009, 204: 125-130.
纳米结构 Ni-Al2O3梯度涂层的制备及结构表征
Arash YAZDANI1,2, Taghi ISFAHANI3
1. Materials Science and Engineering Program, University of California, San Diego, 9500 Gilman Dr., La Jolla, CA 92093-0418, USA;
2. Department of Mechanical and Aerospace Engineering, University of California, San Diego, La Jolla, CA 92093-0411, USA;
3. Metallurgy & Materials Engineering Department, Golpayegan University of Technology, P. O. Box 87717-65651, Golpayegan, Iran
摘 要:将微米级的Ni和Al2O3粉末通过行星式球磨机沉积在固定于球磨罐内顶端的铝板上,制备纳米结构的Ni-Al2O3复合涂层。共采用五组不同Ni与Al2O3质量比(1:1~16:1)的混合粉末,并制备一组纯Ni涂层。用XRD、SEM 和TEM 技术对涂层的相组成和微观结构进行表征。结果表明,初始混合粉末的成分对涂层中Al2O3颗粒的含量和微观结构有很大影响。Al2O3含量高的初始混合粉沉积的涂层中,Al2O3颗粒所占体积分数大。虽然通过本研究的方法可沉积出含有50% Al2O3颗粒的Ni-Al2O3复合涂层,但其性能差,制备出的致密度高、无裂纹和/或孔洞的涂层中Al2O3颗粒的含量小于20%,且需用Ni和Al2O3质量比≥4:1 的初始混合粉制备。研究还发现,机械和冶金结合是涂层和铝基体结合的主要机理。通过在同一制备条件下沉积两种独立的涂料层可以成功制备出具有较好的致密度和完整性的功能梯度复合涂层。
关键词:金属基复合物;Ni-Al2O3梯度涂层;结构表征
(Edited by Wei-ping CHEN)
Corresponding author: Arash YAZDANI; Tel/Fax: +1-801-5875067; E-mail: ayazdani@eng.ucsd.edu
DOI: 10.1016/S1003-6326(18)64640-0
Abstract: Powder charges of micron-size Ni and Al2O3 were utilized to deposit nano-structured Ni-Al2O3 composite coatings on an aluminum plate fixed at the top end of a milling vial using a planetary ball mill. Composite coatings were fabricated using powder mixtures with a wide range of Ni/Al2O3 mass ratio varying from 1:1 to plain Ni. XRD, SEM and TEM techniques were employed to study the structural characteristics of the coatings. It was found that the composition of the starting mixture strongly affects the Al2O3 content and the microstructure of the final coating. Mixtures containing higher contents of Al2O3 yield higher volume fractions of the Al2O3 particles in the coating. Though Ni-Al2O3 composite coatings with about 50% of Al2O3 particles were successfully deposited, well-compacted and free of cracks and/or voids coatings included less than 20% (volume fraction) of Al2O3 particles which were deposited from powder mixtures with Ni/Al2O3 mass ratios of 4:1 or higher. Moreover, mechanical and metallurgical bondings are the main mechanisms of the adhesion of the coating to the Al substrate. Finally, functionally graded composite coatings with noticeable compaction and integrity were produced by deposition of two separate layers under identical coating conditions.


