CaCO3对硅酸三钙水化性能的影响
肖佳,勾成福,金勇刚,邢昊
(中南大学 土木建筑学院,湖南 长沙,410075)
摘 要:
摘 要:通过测定水化产物中的Ca(OH)2含量、化学结合水,借助X线衍射分析(XRD)、热重-差热分析(TG-DSC)、红外光谱分析(IRS)和量热分析,研究CaCO3对硅酸三钙(C3S)水化速度和水化产物的影响。研究结果表明:CaCO3促进硅酸三钙(C3S)早期水化,阻碍C3S的后期水化;CaCO3的加入并未导致水化产物新相的生成而主要使C3S水化速度发生改变,使C3S水化的第一放热峰比纯C3S的放热峰明显增高、前移和变窄,CaCO3加快C3S的放热速度;CaCO3掺量愈高,C3S早期水化愈快。
关键词:
中图分类号:TU503 文献标志码:A 文章编号:1672-7207(2010)05-1894-06
Effects of CaCO3 on hydration performance of C3S
XIAO Jia, GOU Cheng-fu, JIN Yong-gang, XING Hao
(School of Civil Engineering and Architecture, Central South University, Changsha 410075, China)
Abstract: The effects of CaCO3 on the hydration process and the hydration products of C3S were studied by X-ray diffraction (XRD), thermo gravimetric-differential scanning calorimetry (TG-DSC), infrared spectrum (IRS), calorimetric analysis and the measurement of Ca(OH)2 and chemically bound water content of the hydration products. The experimental results indicate that the addition of CaCO3 can promote the earlier hydration of C3S without any new hydration product. The first exothermic peak of the sample containing CaCO3 appears in advance and is higher and narrower than that of the pure sample.
Key words: 3CaO?SiO2 (C3S); CaCO3; hydration performance; effect mechanism
石灰石作为水泥生产原料和混凝土的粗、细骨料,在其开采过程中产生了大量的石屑和石灰石粉。将其用作水泥工业的混合材和混凝土的矿物掺合料,在技术、经济和生态方面具有潜在优势[1-4]。因而,在水泥混凝土中充分和合理地应用石灰石粉,可以达到节能和废物利用的目的,具有技术经济和环保意义。水泥是由具有不同化学特性的矿物组成,其水化是一个复杂的多相化学反应过程,石灰石粉或CaCO3作用下的水泥水化更增加了其复杂性。国内外主要开展了石灰石粉或CaCO3对水泥混凝土的作用机理和应用研究[1-10],主要表现在减少新拌混凝土的泌水和离析现象,并作为掺合料在自密实混凝土广泛应用。研究表明[11-15],石灰石粉或CaCO3主要是对水泥中熟料矿物C3S和C3A的水化及其生成产物产生了影响,认为其加速了C3S水化,提高早期强度,但对水泥混凝土长期性能不利。众多的研究结果有很多相互矛盾之处,缺少深入的机理研究。工程调研也发现了很多石灰石粉应用不当的案例,这都是由于缺乏对石灰石粉或CaCO3对水泥混凝土作用机理研究而导致的。在此,本文作者通过测定水化产物中的Ca(OH)2含量、化学结合水,借助XRD,TG-DSC,IRS和量热分析,讨论CaCO3对水泥熟料主要矿物C3S水化速度和水化产物的影响。
1 实验
1.1 原材料
1.1.1 C3S
用分析纯的CaCO3和SiO2为原料,由实验室高温合成C3S。所得C3S的X线衍射谱如图1所示。C3S的比表面积(氮吸附法)为580 m2/kg,C3S和CaCO3的粒径如表1所示。C3S颗粒的扫描电镜形貌如图2所示。由图2可见:C3S颗粒具有不规则的几何形状,有棱角且大小不一致。

图1 C3S 矿物的XRD谱
Fig.1 XRD pattern of C3S
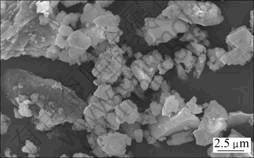
图2 C3S的SEM像
Fig.2 SEM image of C3S
1.1.2 CaCO3
CaCO3(化学纯试剂)的比表面积(氮吸附法)为 4 580 m2/kg,粒径如表1所示。CaCO3颗粒的扫描电镜形貌如图3所示。从图3可见:CaCO3颗粒为薄片,大多数具有近似于长椭圆状,少数具有不规则形状,片状晶体互相堆积在一起,呈团状。
表1 C3S和CaCO3的粒径
Table 1 Particle size of C3S and CaCO3

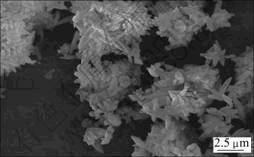
图3 CaCO3的SEM像
Fig.3 SEM image of CaCO3
1.2 试验方法
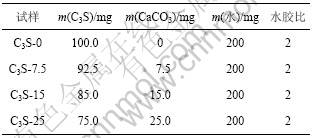
用CaCO3等量取代C3S,C3S-CaCO3-H2O 体系试验配合比见表2。按表2配制好净浆并放于标准养护室中养护,到试验龄期时取出用乙醇终止水化,将试样磨细制成粉末并通过孔径为80 μm的筛,烘干后密封保存以供XRD,TG-DSC和IRS分析使用。C3S-CaCO3-H2O 体系量热分析试验配合比见表3。
表2 C3S-CaCO3-H2O 体系配合比
Table 2 Mix proportions of C3S-CaCO3-H2O system
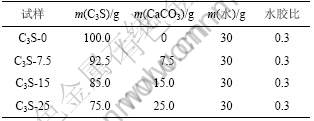
表3 C3S-CaCO3-H2O 体系量热分析配合比
Table 3 Mix proportions of calorimetric analysis of C3S-CaCO3-H2O system
2 结果与讨论
2.1 C3S-CaCO3-H2O体系水化速度
2.1.1 Ca(OH)2含量分析
C3S-CaCO3-H2O体系的C3S-0和C3S-15这2种试样Ca(OH)2含量随水化龄期的变化如图4所示。由图4可见:随着水化龄期的增长,2种试样Ca(OH)2的含量都随之增加,表明纯C3S和掺有CaCO3的C3S水化度均随着水化时间的增长而增加;掺入15% CaCO3,使C3S水化1,3和7 d的Ca(OH)2含量分别比纯C3S同期水化的Ca(OH)2含量增加38.4%,51.8%和72.4%;28 d的Ca(OH)2含量比同期纯C3S的Ca(OH)2含量减少28.0%。该试验结果初步证明CaCO3促进了C3S早期水化,而阻碍了其后期水化。
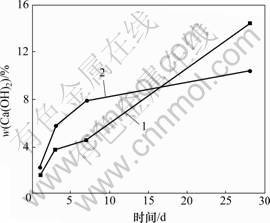
1—C3S-0; 2—C3S-15
图4 C3S中Ca(OH)2含量随水化龄期的变化
Fig.4 Variations of content of Ca(OH)2 with hydration age
2.1.2 化学结合水量分析
C3S-CaCO3-H2O体系试样各龄期化学结合水含量如图5所示。掺有15% CaCO3的C3S化学结合水含量与纯C3S含量相比,当水化1,3和7 d时,分别增加147.1%,17.3%和6.7%;当水化28 d时,减少32.8%。体系的化学结合水含量同样表明:CaCO3促进了C3S的早期水化,妨碍了其后期水化。
2.1.3 量热分析
图6所示为C3S-CaCO3-H2O体系试样的量热曲线。从图6可见:15%和25% CaCO3的掺入使C3S的放热峰比纯C3S的放热峰明显增高、前移和变窄,CaCO3明显加快了C3S的放热速度;CaCO3掺量愈高,C3S的放热速度愈快。CaCO3对C3S的早期水化产生了有利影响。

1—C3S-0; 2—C3S-15
图5 C3S中化学结合水含量随水化龄期的变化
Fig.5 Variations of chemically bound water content with hydration age
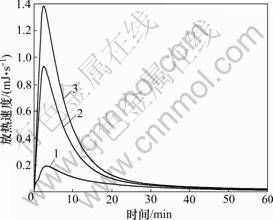
1—C3S; 2—C3S-15; 3—C3S-25
图6 C3S-CaCO3-H2O体系试样的量热曲线
Fig.6 Calorimetric curve of samples of C3S-CaCO3-H2O system
通过对C3S-CaCO3-H2O体系试样的Ca(OH)2含量、化学结合水和量热分析,得出CaCO3促进C3S早期水化,阻碍其后期水化的规律。对C3S早期水化的加速作用主要归因于CaCO3的稀释效应、微晶核效应和分散效应。一方面,CaCO3的掺入导致浆体有效水灰比增大,可供C3S水化的水量增多,促使了C3S早期水化;另一方面,CaCO3颗粒对C3S水化有明显的微晶核效应[16],当C3S开始水化时,释放了大量的Ca2+。当Ca2+扩散到CaCO3颗粒表面附近时,根据吸附理论,CaCO3对Ca2+产生了物理吸附作用并为Ca(OH)2优先成核提供了大量初始形核点,加快了Ca(OH)2的形成,导致水化浆体中C3S颗粒周围Ca2+浓度降低,从而使C3S水化加速。此外,CaCO3和C3S颗粒的平均粒径分别为5.81 μm和22.71 μm,早期浆体中超细的CaCO3颗粒改善了C3S颗粒分布状况,对C3S颗粒起到了分散作用,使C3S颗粒与水接触的面积增大,促使C3S水化加速。而纯C3S水化浆体中,C3S颗粒之间包裹了一部分水,也阻碍了早期C3S的水化。
随着体系水化的进行,水化产物逐渐增加,一方面,水化后期CaCO3颗粒上已附着有Ca(OH)2晶 体,提供成核点的CaCO3减弱了对Ca2+的物理吸附作用;另一方面,超细的CaCO3颗粒比表面积大,容易黏附在C3S颗粒上,CaCO3对C3S水化早期的分散效应逐渐转变成对C3S的屏蔽效应,影响和阻碍了C3S水化后期离子的扩散,使C3S的进一步水化减缓。而纯C3S水化浆体中,随着水化的进行,C3S颗粒之间包裹的一部分水逐渐释放出来,促使了C3S后期的 水化。
2.2 C3S-CaCO3-H2O体系水化产物
2.2.1 XRD分析
通过分析C3S-CaCO3-H2O体系的C3S-0和C3S-15这2种试样水化1,3,7和28 d的XRD谱(见图7)得出:纯C3S的水化产物是Ca(OH)2和C-S-H凝胶,掺有15% CaCO3的C3S-15试样的水化产物也是Ca(OH)2和C-S-H凝胶,没有发现新相形成。对掺有7.5% CaCO3的C3S-7.5试样进行XRD分析,发现其水化产物同样是Ca(OH)2和C-S-H凝胶。
2.2.2 DSC分析
图8(a)和8(b)所示分别为C3S-CaCO3-H2O体系的C3S-0和C3S-15这2种试样的DSC谱。在图8(a)中,62~78 ℃之间的吸热峰为C-S-H凝胶脱水产生,420~440 ℃之间的峰为Ca(OH)2脱水的吸热峰,675~680 ℃之间的峰(28 d)为CaCO3分解产生的吸热峰,纯C3S水化28 d时出现了轻微碳化现象。通过DSC分析可知:纯C3S水化的产物为Ca(OH)2和C-S-H凝胶。与图8(a)比较,图8(b)中只在710~720 ℃之间增加了吸热峰,该峰为CaCO3的分解峰,掺有15% CaCO3的C3S-15试样水化没有发现新相形成。
2.2.3 IRS分析
图9(a)和9(b)所示分别为C3S-CaCO3-H2O体系的C3S-0和C3S-15这2种试样各水化龄期的IRS谱。
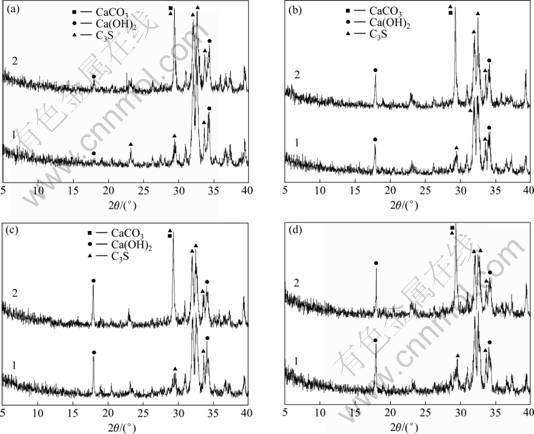
试样:1—C3S-0;2—C3S-15
水化龄期/d: (a) 1; (b) 3; (c) 7; (d) 28
图7 C3S-CaCO3-H2O体系试样的XRD谱
Fig.7 XRD patterns of samples of C3S-CaCO3-H2O system
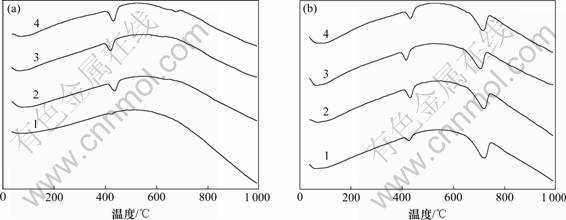
(a) C3S-0; (b) C3S-15
水化龄期/d: 1—1; 2—3; 3—7; 4—28
图8 C3S-CaCO3-H2O体系试样的DSC谱
Fig.8 DSC patterns of samples of C3S-CaCO3-H2O system
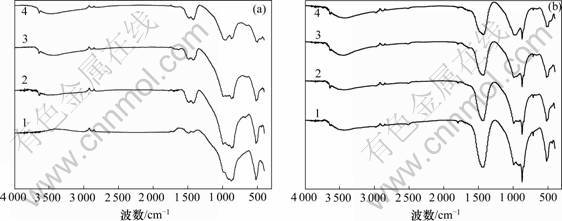
(a) C3S-0; (b) C3S-15
水化龄期/d: 1—1; 2—3; 3—7; 4—28
图9 C3S-CaCO3-H2O体系试样的IRS谱
Fig.9 IRS patterns of samples of C3S-CaCO3-H2O system
C3S-15试样新增加的波数是2 515,1 795~1 796,1 438~1 440,872和713 cm-1,与纯CaCO3的红外光谱对比,可以判断是CaCO3的官能团CO32-产生的峰。C3S-0试样水化28 d出现了波数871 cm-1,是其Ca(OH)2有少许碳化的缘故,这与其DSC的分析结果相同。C3S-15试样的各龄期IRS谱中未发现新相形成。
通过对C3S-CaCO3-H2O体系XRD,TG-DSC和IRS分析可知:CaCO3的掺入并未导致水化产物新相的生成。
3 结论
(1) CaCO3促进了C3S早期水化,阻碍了C3S的后期水化。
(2) CaCO3的加入并未导致水化产物新相的生成而主要使C3S水化速度发生了改变,使C3S水化的第一放热峰比纯C3S的放热峰明显增高、前移和变窄,CaCO3加快了C3S的放热速度;CaCO3掺量愈高,C3S早期水化愈快。
参考文献:
[1] Voglis N, Kakali G, Chaniotakis E, et al. Portland-limestone cements. Their properties and hydration compared to those of other composite cements[J]. Cement and Concrete Composites, 2005, 27(2): 191-196.
[2] Carrasco M F, Menéndez G, Bonavetti V, et al. Strength optimization of “tailor-made cement” with limestone filler and blast furnace slag[J]. Cement and Concrete Research, 2005, 35 (7): 1324-1331.
[3] Kakali G, Tsivilis S, Aggeli E, et al. Hydration products of C3A, C3S and Portland cement in the presence of CaCO3[J]. Cement and Concrete Research, 2000, 30(7): 1073-1077.
[4] Bonavetti V L, Rahhal V F, Irassar E F. Studies on the carboaluminate formation in limestone filler-blended cements[J]. Cement and Concrete Research, 2001, 31(6): 853-859.
[5] Yahia A, Tanimura M, Shimoyama Y. Rheological properties of highly flowable mortar containing limestone filler-effect of powder content and W/C ratio[J]. Cement and Concrete Research, 2005, 35(3): 532-539.
[6] Zeli? J, Krstulovi? R, Tkal?ec E, et al. The properties of Portland cement-limestone-silica fume mortars[J]. Cement and Concrete Research, 2000, 30(1): 145-152.
[7] Bosiljkov V B. SCC mixes with poorly graded aggregate and high volume of limestone filler[J]. Cement and Concrete Research, 2003, 33(9): 1279-1286.
[8] Bonavetti V, Donza H, Rahhal V, et al. Influence of initial curing on the properties of concrete containing limestone blended cement[J]. Cement and Concrete Research, 2000, 30(5): 703-708.
[9] 杨华山, 方坤河, 涂胜金, 等. 石灰石粉在水泥基材料中的作用及其机理[J]. 混凝土, 2006(6): 32-35.
YANG Hua-shan, FANG Kun-he, TU Sheng-jin, et al. The effect and its mechanism of calcium carbonate on the cement based materials[J]. Concrete, 2006(6): 32-35.
[10] 肖佳, 邓德华, 唐咸燕, 等. 粉煤灰改善石灰石粉混凝土氯离子扩散性能研究[J]. 中南林业科技大学学报, 2007, 27(5): 79-82.
XIAO Jia, DENG De-hua, TANG Xian-yan, et al. Effects of fly ash on the chloride diffusivity of concrete with limestone powder[J]. Journal of Central South University of Forestry & Technology, 2007, 27(5): 79-82.
[11] 孔祥芝. 石灰石粉作水工碾压混凝土掺合料的研究[D]. 北京: 中国水利水电科学研究院, 2006: 59-70.
KONG XIANG-zhi. Research on limestone powder as roller-compacted concrete additive[D]. Beijing: China Institute of Water Resources and Hydropower Research, 2006: 59-70.
[12] 马烨红. 石灰石粉作混凝土矿物掺合料的研究[D]. 广州: 华南理工大学材料学院, 2007: 50-60.
MA Ye-hong. Study on limestone powder used as mineral admixture of concrete[D]. Guangzhou: South China University of Technology. School of Materials, 2007: 50-60.
[13] 肖佳, 邓德华, 唐咸燕, 等. 矿渣和石灰石粉双掺对混凝土抗氯离子渗透性能影响的试验[J]. 工业建筑, 2007, 37(10): 73-75, 87.
XIAO Jia, DENG De-hua, TANG Xian-yan, et al. Experiment on the anti-chloride ion permeability of concrete mixed with slag and limestone powder[J]. Industrial Construction, 2007, 37(10): 73-75, 87.
[14] ZHANG Yong-juan, ZHANG Xiong. Research on effect of limestone and gypsum on C3A, C3S and PC clinker system[J]. Construction and Building Materials, 2007, 22(8): 1634-1642.
[15] 肖佳, 王永和, 邓德华, 等. 粉煤灰-石灰石粉高强混凝土的Cl-扩散性能[J]. 建筑材料学报, 2008, 11(2): 212-216.
XIAO Jia, WANG Yong-he, DENG De-hua, et al. Chloride diffusivities of high strength concrete with fly ash and ground limestone[J]. Journal of Building Materials, 2008, 11(2): 212-216.
[16] 陆平, 陆树标. CaCO3对C3S水化的影响[J]. 硅酸盐学报, 1987, 15(4): 289-294.
LU Ping, LU Shu-biao. Effect of calcium carbonate on the hydration of C3S[J]. Journal of the Chinese Ceramic Society, 1987, 15(4): 289-294.
收稿日期:2010-04-23;修回日期:2010-07-08
基金项目:国家自然科学基金资助项目(50678177)
通信作者:肖佳(1964-),女,湖南衡阳人,博士,教授,从事高性能混凝土和新型建筑材料研究;电话:13974842678;E-mail: jiaxiao@mail.csu.edu.cn


