Cross-sectional analysis on microstructure of plasma-sprayed HA+TiO2 composite coatings on titanium substrate
Lǚ Yu-peng(吕宇鹏)1, LI Mu-sen(李木森)1, WANG Jian-hua(王建华)2,
SUN Rui-xue(孙瑞雪)1, LI Shi-tong(李士同)1, ZHU Rui-fu(朱瑞富)1
(1. School of Materials Science and Engineering;
2. School of Stomatology, Shandong University, Jinan 250062, China)
Abstract:
The cross-sectional analysis on hydroxyapatite (HA) coating and HA+TiO2 composite coating was conducted by using electron probe microanalyser (EPMA). The results reveal that annealing at 650℃ leads to the cracking within the HA coating or along the coating/substrate interface. The ribbon-like regions in HA coating are verified to contain less PO4-4 groups resulted from the high temperature melting of HA particles in plasma flame. From the viewpoint of microstructural observation, it can be concluded that the addition of TiO2 into HA coating can effectively strengthen and toughen the whole coating system with a shift of the well-bonded interface from the THA (top HA) coating/HTBC (HA+TiO2 bond coat) interface in the as-sprayed THBC (top HA-HTBC) coating to the HTBC/Ti substrate interface in the heat treated THBC coating. The THA coating bonds well to Ti substrate perhaps via its TiO2 hobnobbing with the Ti oxides formed on the Ti substrate.
Key words:
cross-sectional analysis; hydroxyapatite; composite coating; plasma spraying CLC number: R318.08;
Document code: A
1 INTRODUCTION
Hydroxyapatite(HA) has been extensively studied and clinically applied, especially as a bioactive coating on metal implants. The positive effect of HA coating on implants, such as more rapid and stable fixation of the implant to bone, stronger bonding between the bone and the implant, increased uniform bone in-growth at the bone-implant interface and decreased release of metal ions from the implant to the body, has been widely reported. However, there is controversy as to its long-term effectiveness and performance. Therefore, many studies have been carried out in order to improve the interfacial bond strength and coating duration of the HA-titanium implant materials system. Thermally sprayed gradient coatings[1-6], composite coatings[7-12] and bonding interlayer[13-16] were adopted with incorporation of Ti, titania(TiO2) and zirconia(ZrO2) as reinforcing additives. Most researches show that the adhesive and cohesive strengths can be further increased. In the case of TiO2 selected to be incorporated, its favorable biological effects and high corrosion resistance were usually considered[11, 12, 17]. In reality, TiO2 should substantially possess a mechanical advantage over other materials in incorporating into HA coating.
HA coating endows the implant not only with bioactivity but also with a protective layer against the release of metal ions. In order to increase the coating duration after implantation, it is necessary to perform heat treatment to restore the crystallinity and OH- content of as-sprayed HA coating with amorphous and decomposed phases[18-20]. It was reported that HA coating with a lower crystallinity had a higher bioactivity[21] and its initial dissolution was a part of the events leading to bone tissue growth enhancement and bone tissue bonding[22]. On the contrary, the studies in vitro and in vivo showed that HA coatings with higher crystallinity yielded higher rates of cell proliferation[23], and there was no difference in the percentage of the as-deposited and annealed coatings, to which bone was found to be apposed by ESEM imaging after 3 and 10 days[24]. It was suggested that implants in trabecular or cancellous bone would require coatings with a very high crystallinity[25].
In this study, cross-sectional analysis on microstructure of plasma-sprayed HA coating and HA+TiO2 composite coating on titanium substrate before and after heat treatment has been conducted. Attempts are made to probe into the effects of heat treatment and addition of TiO2 on structure of the HA coating.
2 MATERIALS AND METHODS
2.1 Specimen fabrication
Fully crystallized 10-20μm (fine) and 60-80μm (coarse) HA powders (Sichuan University, China) and 50-60μm rutile TiO2 powders(Shen- yang Grind Wheel Plant, China) were obtained commercially and were used as the starting materials. The composite powders consisting of 50% (volume fraction) HA (60-80μm) +50%TiO2 for forming the interlayer were prepared through a mechanical-mixing process in a ball mill pulverizer. Commercially pure titanium buttons, with a diameter of 13mm and a thickness of 2mm, were used as the substrate materials. Prior to plasma spraying, all the substrate surfaces were sandblasted with Al2O3 grit of size 500μm. Under the same spraying condition, two kinds of specimens, i.e. the pure HA coating and the two-layer one consisting of the HA top coating and the HA+TiO2 interlayer (THBC coating) on titanium, were produced by plasma spraying using a Sulzer Metco 9M equipment. Argon and hydrogen were used as the plasma arc gases and argon as the powder carrier gas. The arc current and voltage were 500A and 60V respectively. The powder feed rate was 30-40g/min and the spray distance was 100mm. The post-heat-treatment of the coatings was carried out in air at 650℃ for 120min.
2.2 Microstructural characterization
Considering the brittleness of the coatings, the cross-sections of the as-sprayed and heat treated coatings were gently polished to prepare the metallographic samples. A JXA-8800R electron probe microanalyser (EPMA) with a Link ISIS300 energy spectrum analyser was used to give the back scattering electron (BSE) image and determine element composition of the coatings. The surface of samples was sputter-coated with carbon.
3 RESULTS
3.1 Effect of heat treatment on coating and interface microstructure
Fig.1 shows the cross-sectional observation on coarse HA coating before and after annealing at 650℃. It can be seen that the polished section of the as-sprayed HA coating is smooth with some pores, fine cracks and ribbon-like regions (Fig.1(a)). From Fig.1(b) it can be clearly found that the heat treatment induces the cracking of HA coating and the broadening of original cracks. Fig.2 shows the cross-sectional observation on coarse HA coating/titanium interface before and after heat treatment. Similarly, the heat treatment causes the HA coating to be separated from the titanium substrate (Figs.2(a-d)). From both Fig.1 and Fig.2, it is shown that the heat treatment can eliminate the ribbon-like regions appearing in the as-sprayed HA coating to a great extent. To understand the formation and composition of the ribbon-like regions, we conducted the element analysis on them as below.
3.2 Chemical inhomogeneity of HA coating
Fig.3 shows the BSE image of the as-sprayed fine HA coating and the corresponding plane distributions of Ca, P and O. The HA coating made of fine powder contains more ribbon-like regions, compared with the coarse HA coating (see Fig.1(a) and Fig.2(b)). The detailed element analysis (Figs.3(b-d)) indicates that the HA coating exhibits chemical inhomogeneity, i.e. less P and O content in the composition of the ribbon-like regions, is different from the other regions, which may be caused by the more loss of PO4-4 groups.
3.3 Microstructure of HA+TiO2 composite coating
Fig.4 shows the cross-sectional pictures of the as-sprayed THBC coating with EDS analysis. It can be seen that the HA+TiO2 bonding interlayer (HTBC) very well adheres to the top HA coating (THA coating), but there exists a separation between the HTBC and Ti substrate presumably due to the damage during preparation. Within the HTBC, BSE images from the polished cross-sections clearly demonstrate the HA and TiO2 phases lay ered in an alternating pattern (Figs.4(b) and (c)). EDS analysis indicates that the dark gray area in the HTBC is HA and the bright gray area is TiO2. The EDS spectra shown in Fig.4(d) also indicate the coexistence of Ca, P and Ti in some micro- regions. It is found that there are a few cracks in the THA coating, in which HA is continuous with that in the HTBC (Fig.4(b)). In addition, HA and TiO2 splats bond to each other very well within the HTBC.
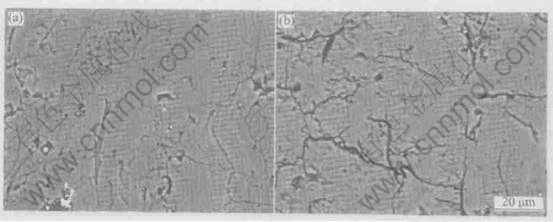
Fig.1 Cross-sectional observations on coarse HA coating before (a) and after (b) annealing at 650℃
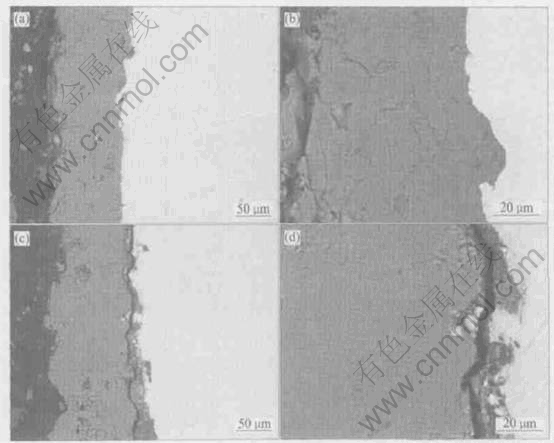
Fig.2 Cross-sectional observations on coarse HA coating/titanium interface before (a, b) and after (c, d) annealing at 650℃
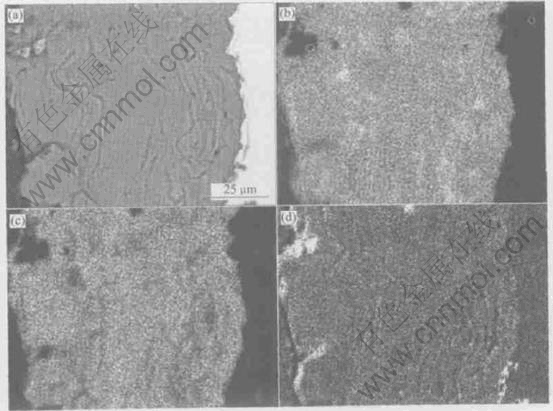
Fig.3 BSE image of as-sprayed fine HA coating (a) and corresponding plane distributions of Ca(b), P(c) and O(d)
Fig.5 shows the cross-sectional pictures of the heat-treated THBC coating. Compared with the as sprayed THBC coating, the HTBC in the heat-treated THBC coating adheres more tightly to the Ti substrate and the local separation takes place along the THA coating/HTBC interface rather than along the HTBC/Ti substrate interface. At the same time, more cracks approximatively perpendicular to the interface appear in the THA coating (Figs.5(b-d)). However, less cracks form in the HTBC (Fig.5(b-d)).
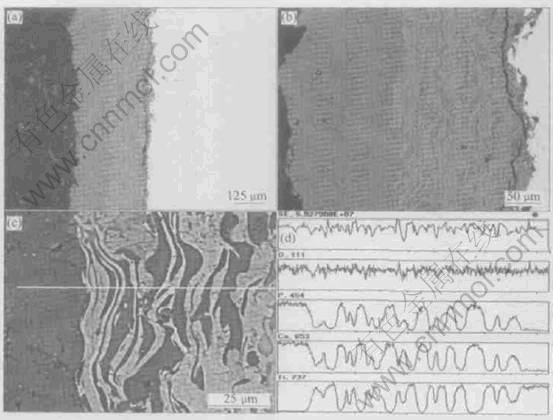
Fig.4 BSE images of cross-sections of as-sprayed THBC coating with different magnifications (a-c) and EDS line analysis profiles (d) for (c)
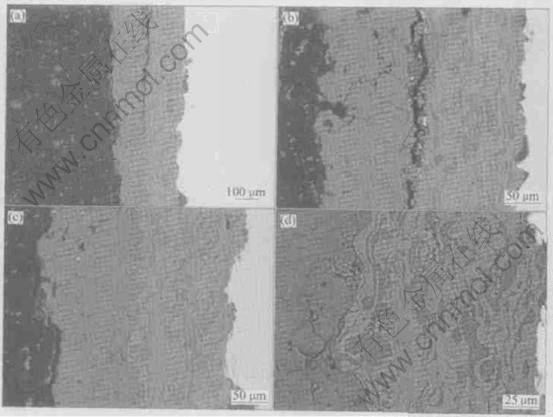
Fig.5 BSE images of cross-sections of post-spray heat-treated THBC coating at different locations and magnifications
4 DISCUSSION
Cross-sectional analysis is a more useful method to examine the inner structural changes of coating than surface analysis. Fig.1 and Fig.2 indicate that the heat treatment causes more pronounced cracking within HA coating and at coating/substrate interface. This is caused by an additional increment of tensile stress resulting from the transformation of various non-HA phases, including amorphous phase, to the crystalline HA with a volume shrinkage[19].
The element analysis shown in Fig.3 indicates that the ribbon-like regions contain less P and O, which means the loss of PO4-4 group resulting from HA transforming into amorphous phase and other non-HA phases caused by the high temperature melting of HA particles in plasma flame. These non-HA phases may have negative effect on coating performances and the heat treatment can restore the HA structure by transforming non-HA phases into HA. The finer HA particles can be melted more sufficiently than the coarser ones, so that more ribbon-like regions exist in the fine HA coating than in the coarse HA coating (see Figs.1-3).
Fig.4(a) and Fig.5(a) show that in the as-sprayed THBC coating, the HTBC adhering well to THA coating is completely separated from the Ti substrate, while in the heat treated THBC coating the HTBC adhering well to the Ti substrate is partially separated from the THA coating. As mentioned by other researchers, this separation is presumably due to the damage during preparation. In any case, where the separation occurs indicates where a relatively weaker interface is located. As a result, it can be deduced that there is a shift of the relatively tighter bonding from the HAT coating/HTBC interface in the as-sprayed THBC coating to the HTBC/Ti substrate interface in the heat treated THBC coating. Generally, the cross-sectional analysis shows that the addition of TiO2 strengthens the HTBC, perhaps resulting in strengthening the THBC coating system. This is more pronounced for the heat-treated THBC coating (Fig.5).
According to a previous research[26], except its strengthening effect on HA, the reasons why TiO2 is employed in the present study as an addition into HA coating are as follows. (1) TiO2 is biocompatible and it is thought to endow Ti implant with good biocompatibility. (2) The melting point of TiO2 is much lower than that of Al2O3, ZrO2, Y2O3, etc, which are also biocompatible and have already been used to enhance HA. (3) TiO2 is expected to integrate with Ti substrate through a thin TiO2 film which can be naturally formed on the surface of Ti substrate.
The toughening and strengthening mechanisms for the THBC coating and its constituent parts are hence suggested to be due to the following aspects. (1) In the HTBC, the elongated TiO2 particles along the direction parallel to the interface act as the obstacles for pinning the cracks or deviating the crack path from its original direction. Meanwhile, the stress-induced microcracking generated by thermal expansion or elastic mismatch would contribute to the reduction of the near-tip stresses. (2) TiO2 of the HTBC is suggested to enhance the bonding between the HTBC and the Ti substrate through its affinity with the Ti oxide on the surface of Ti substrate. (3) In comparison with pure HA coating on Ti, the THA coating of THBC system is thought to be toughened by the interposition of HTBC with an in-between CTE of HA and TiO2, by which the CTE mismatch between HA and Ti and the residual tensile stress in the HAT coating can be reduced.
5 CONCLUSIONS
The cross-sectional analysis on HA coating and HA+TiO2 coating shows that annealing at 650℃ leads to the cracking within the HA coating or along the coating/substrate interface. The ribbon-like regions in the HA coating are verified to contain less PO4-4 groups resulted from the high temperature melting of HA particles in the plasma flame. The addition of TiO2 into HA coating may strengthen and toughen the whole coating system. The cross-sectional observations confirm that there is a shift of the well bonding interlayer from the THA coating/HTBC interface in the as-sprayed THBC coating to the HTBC/Ti substrate interface in the heat treated THBC coating. The THA coating bonds well to Ti substrate perhaps via its TiO2 hobnobing with the Ti oxides formed on the Ti substrate.
REFERENCES
[1]Watari F, Yokoyama A, Saso F, et al. Fabrication and properties of functionally graded dental implant [J]. Composites Part B, 1997, 28B: 5-11.
[2]Kyeck S, Remer P. Realisation of graded coatings for biomedical use [J]. Mater Sci Forum, 1999, 308-311: 368-373.
[3]Park E, Condrate Sr R A. Graded coating of hydroxyapatite and titanium by atmospheric plasma spraying [J]. Mater Lett, 1999, 40: 228-234.
[4]Kumar R R, Maruno S. Functionally graded coatings of HA-G-Ti composites and their in vivo studies [J]. Mater Sci Eng, 2002, A334: 156-162.
[5]Kumar R R, Wang M. Functionally graded bioactive coatings of hydroxyapatite/titanium oxide composite system [J]. Mater Lett, 2002, 55: 133-137.
[6]Khor K A, Gu Y W, Quek C H, et al. Plasma spraying of graded hydroxyapatite/Ti-6Al-4V coatings [J]. Surface and Coatings Technol, 2003, 168: 195-201.
[7]Quek C H, Khor K A, Cheang P. Influence of processing parameters in the plasma spraying of hydroxyapatite/Ti-6Al-4V composite coatings [J]. J Mater Processing Technol, 1999, 89-90: 550-555.
[8]Chou B Y, Chang E. Microstructural characterization of plasma-sprayed hydroxyapatite- 10wt.% ZrO2 composite coating on titanium[J]. Biomaterials, 1999, 20: 1823-1832.
[9]Zheng X B, Huang M H, Ding C X. Bond strength of plasma-sprayed hydroxyapatite/Ti composite coatings [J]. Biomaterials, 2000, 21: 841-849.
[10]Khor K A, Fu L, Lim V J P, et al. The effects of ZrO2 on the phase compositions of plasma-sprayed HA/YSZ composite coatings [J]. Mater Sci Eng, 2000, A276: 160-166.
[11]Li H, Khor K A, Cheang P. Titanium dioxide reinforced hydroxyapatite coatings deposited by high velocity oxy-fuel (HVOF) spray [J]. Biomaterials, 2002, 23; 85-91.
[12]Li H, Khor K A, Cheang P. Impact formation and microstructure characterization of thermal sprayed hydroxyapatite/titania composite coatings [J]. Biomaterials, 2003, 24: 949-957.
[13]Lamy D, Pierre A C, Heimann R B. Hydroxyapatite coatings with a bond coat of biomedical implants by plasma projection [J]. J Mater Res, 1996, 11: 680-686.
[14]Kurzweg H, Heimann R B, Troczynski T, et al. Development of plasma-sprayed bioceramics coatings with bond coats based on titania and zirconia [J]. Biomaterials, 1998, 19: 1507-1511.
[15]Chou B Y, Chang E. Interface investigation of plasma-sprayed hydroxyapatite coating on titanium alloy with ZrO2 intermediate layer as a bond coat [J]. Scripta Materialia, 2001, 45: 487-493.
[16]Chou B Y, Chang E. Plasma-sprayed zirconia bond coat as an intermediate layer for hydroxyapatite coating on titanium alloy substrate [J]. J Mater Sci: Mater Med, 2002, 13: 589-595.
[17]Nie X, Leyland A, Matthews A. Deposition of layered bioceramics hydroxyapatite/TiO2 coatings on titanium alloys using a hybrid technique of micro-arc oxidation and electrophoresis [J]. Surface and Coatings Technol, 2000, 125: 407-414.
[18]Zyman Z, Weng J, Liu X, et al. Phase and structural changes in hydroxyapatite coatings under heat treatment [J]. Biomaterials, 1994, 15: 151-155.
[19]Tsui Y C, Doyle C, Clyne T W. Plasma sprayed hydroxyapatite coatings on titanium substrates (Part 2): Optimization of coating properties [J]. Biomaterials, 1998, 19: 2031-2043.
[20]Li H, Khor K A, Cheang P. Properties of heat-treated calcium phosphate coatings deposited by high-velocity oxy-fuel (HVOF) spray [J]. Biomaterials, 2002, 23: 2105-2112.
[21]Shi D, Jiang G, Bauer J. The effect of structural characteristics on the in vitro bioactivity of hydroxyapatite [J]. J Biomed Mater Res (Appl Biomater), 2002, 63: 71-78.
[22]Ducheyne P, Radin S, King L. The effect of calcium phosphate ceramic composition and structure on in vitro behavior. Ⅰ. Dissolution [J]. J Biomed Mater Res, 1993, 27: 25-34.
[23]Chou L, Marek B, Wagner W R. Effects of hydroxyapatite coating crystallinity on biosolubility, cell attachment efficiency and proliferation in vitro [J]. Biomaterials, 1999, 20: 977-985.
[24]Porter A E, Hobbs L W, Rosen V B, et al. The ultrastructure of plasma-sprayed hydroxyapatite-bone interface predisposing to bone bonding [J]. Bio-materials, 2002, 23: 725-733.
[25]Darimont G L, Cloots R, Heinen E, et al. In vivo behavior of hydroxyapatite coatings on tiyanium implants: a quantitative study in the rabbit [J]. Bio-materials, 2002, 23: 2569-2575.
[26]Lu Y P, Li M S, Li S T, et al. Plasma-sprayed hydroxyapatite+titania composite bond coat for hydroxyapatite coating on titanium substrate [J]. Biomaterials, 2004, 25: 4393-4403.
Foundation item: Project (032040105) supported by the Department of Science and Technology of Shandong Province, China
Received date: 2004-03-03; Accepted date: 2004-08-10
Correspondence: L Yu-peng, Associate professor, PhD; Tel: +86-531-8395966; E-mail: Biosdu@sdu.edu.cn


