Trans. Nonferrous Met. Soc. China 27(2017) 925-931
Cascade sulfidation and separation of copper and arsenic from acidic wastewater via gas-liquid reaction
Guo-min JIANG1,3, Bing PENG1,2, Li-yuan CHAI1,2, Qing-wei WANG1,2, Mei-qing SHI2, Yun-yan WANG1,2, Hui LIU1,2
1. School of Metallurgy and Environment, Central South University, Changsha 410083, China;
2. Chinese National Engineering Research Center for Control & Treatment of Heavy Metal Pollution, Changsha 410083, China;
3. Changsha Science Environmental Technology Co., Ltd., Changsha 410000, China
Received 30 May 2016; accepted 10 January 2017
Abstract:
Copper and arsenic in acidic wastewater were separated by cascade sulfidation followed by replacement of arsenic in the precipitates by copper in the solution which was realized by recycling precipitates obtained in the first stage into the initial solution. The effects of reaction time, temperature and H2S dosage on copper and arsenic removal efficiencies as well as the effects of solid-to- liquid ratio, time and temperature on the replacement of arsenic by copper were investigated. With 20 mmol/L H2S at 50 °C within 0.5 min, more than 80% copper and nearly 20% arsenic were precipitated. The separation efficiencies of copper and arsenic were higher than 99% by the replacement reaction between arsenic and copper ions when solid-to-liquid ratio was more than 10% at 20 °C within 10 min. CuS was the main phases in precipitate in which copper content was 63.38% in mass fraction.
Key words:
acidic wastewater; copper; arsenic; cascade sulfidation; separation;
1 Introduction
Many industrial processes, especially in the mining and metallurgical processing industry, discharge acidic effluents contain significant amounts of metals such as copper, nickel, zinc, lead and arsenic [1-4]. Copper is often associated with arsenic in mixed sulphide minerals such as enargite (Cu3AsS4) and tennantite (Cu12As4S13) [5], therefore, among theses heavy metals, Cu and As are found to behave similarly and exist simultaneously in the wastewater [6]. Arsenic contamination has greatly threatened water safety due to its high toxicity and carcinogenicity [7,8]. However, copper in the wastewater is still valuable product that can be recovered from the residual water, which is beneficial to the reuse of the purified water to the production process [9].
The undesirable effects of copper and arsenic can be avoided after treatment prior to discharge [10], by which the recovery of copper can be also achieved. While the first challenge is the effective removal and separation of copper and arsenic [11,12]. In the case of liquid effluents, there are many methods available for the removal of heavy metals: chemical precipitation, adsorption, coagulation, ion-exchange, microbial reduction, and so on [13-21]. On the other hand, dissolved-air flotation was used to recover and separate heavy metals. STALIDIS et al [22] separated copper, zinc and arsenic ions from dilute aqueous solutions by using the dissolved-air technique for the production of fine gas bubbles. It seems to be complex and expensive for the treatment of acidic wastewater. Sulfide precipitation is indeed an effective process for the treatment of toxic heavy metals ions [23,24]. One of the primary advantages of using sulfides is that sulfide precipitation process can achieve a high degree of metal removal over a broad pH range. Metal sulfide sludge also exhibits better thickening and dewatering characteristics than the corresponding metal hydroxide sludge [25]. BHATTACHARYYA et al [26] separated arsenic and other heavy metals by using sodium sulphide. The removals of Cd, Zn and Cu from the actual wastewaters are greater than 99%, and those of As and Se are 98% and >92%, respectively. However, it is H2S that reacts with heavy metals by gas-liquid reaction as sulfur exists almost in the form of H2S rather than S2- and HS- in acid conditions. It takes 5-10 times excess of theoretical amount of sodium sulphide, moreover, a lot of hydrogen sulfide gas escapes, which leads to serious secondary pollution [27].
Gas-liquid sulfidation reaction was proposed in this work to constantly break the balance between hydrogen sulfide and sulfur ions and intensify dynamics process, achieving the high sulfidation efficiency with low cost. H2S was controlled and recycled in a closed system. The objective of this work is to separate copper and arsenic from wastewater by sulfide precipitation and replacement of arsenic in the precipitates by copper in the solution, from which copper is concentrated in the precipitate and recovered. The feasibility of sulfidation and separation of copper and arsenic was analyzed by thermodynamic calculation first. Secondly, the effects of time, temperature and sulfide dosage on copper and arsenic removal efficiencies were investigated, followed by influence of solid-to-liquid ratio, time and temperature on the replacement of arsenic by copper.
2 Experimental
2.1 Reagents
Experiments were carried out with simulated solution. Analytical grade reagents were used in experiments. CuSO4·5H2O and Na2HAsO4·12H2O were used for preparing certain concentrations of the solutions. pH value was adjusted by 0.05 mol/L H2SO4 and 0.1 mol/L NaOH solution. Gaseous sulphide source (H2S) was generated by reaction of FeS and HCl.
2.2 Experimental design
The experimental program was divided into two stages. In the first stage, copper and arsenic were primarily separated by sulfide precipitation. The schematic diagram of the experimental apparatus is shown in Fig. 1.
All experiments were operated at time of 0.5-4 min, temperature of 24-60°C, and sulfide dosage of 0-75 mmol/L in order to investigate the effect of time, temperature and sulfide dosage on removal efficiencies of arsenic and copper, respectively. In the second stage, precipitates obtained in the first stage were recycled to the initial solution to improve the grade of copper in the precipitates through the replacement of arsenic by copper. Investigations on the effect of liquid-to-solid ratio, time and temperature on the removal efficiency were conducted. The removal efficiency was defined by
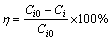 (1)
(1)
where η is the removal efficiency, Ci0 is the copper or arsenic concentration in the initial solution, Ci is the copper or arsenic concentration in the solution after reaction under certain conditions.
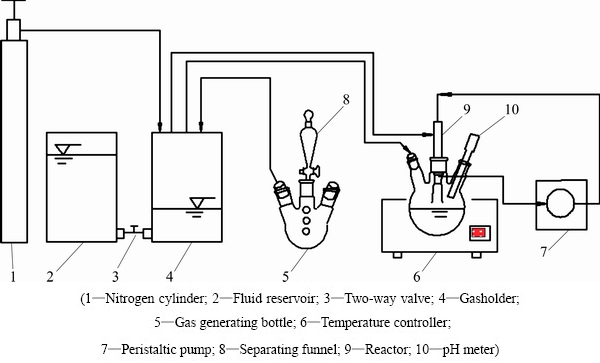
Fig. 1 Schematic diagram of experimental apparatus
2.3 Experimental procedures
Gaseous sulfide source (H2S) generated was collected by displacement of water. Two-way valve was turn off at first. The gas tank was filled with the saturated H2S solution and sealed with paraffin. Gaseous sulfide generated in three-necked flask was then introduced into the tank. Solution in the gasholder was pushed into the fluid reservoir when the two-way valve was opened. A certain volume fraction of H2S gas was obtained by introducing nitrogen into the gasholder when the pressure gauge of cylinder was adjusted to the specified pressure with nitrogen cylinder open. The operating temperature and pH were controlled at a desired level. H2S gas was then delivered to the reactor and the simulated copper sulfate was pumped to the reactor by peristaltic pump.
2.4 Sample collection and analysis
Liquid samples were collected from the reaction system at the end of each reaction under certain conditions. The concentrations of metals in the solutions were determined by inductively coupled plasma spectroscopy (ICP) (iCAP7000), pH measurements were done by a pH meter (PHS-3E). When the sulfidation reaction was finished, PAM was added into the aqueous system to accelerate precipitation and the precipitates were separated by filtration. The precipitates were prepared for analysis by SEM-EDS elemental analysis using scanning electron microscope (SEM) (JSM-6360LV) equipped with energy dispersive X-ray spectrometer (EDX) (EDX-Genesis).
3 Results and discussion
3.1 Feasibility analysis for sulfidation and separation of copper and arsenic
With the introduction of S2-, Cu2+ and As3+ precipitated in the form of CuS and As2S3, respectively. The solubility equilibrium reactions of CuS and As2S3 can be expressed as follows:
CuS Cu2++S2-
Cu2++S2-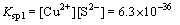 (2)
(2)
As2S3+6H2O 2H3AsO3+3H2S↑
2H3AsO3+3H2S↑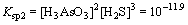 (3)
(3)
The sulfur-containing aqueous solution consists of [H+], [OH-], [H2S], [HS-], [S2-], and the total sulfur ion concentration of the system can be expressed as follows:
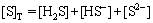 (4)
(4)
The stepwise stability constants for each ion at 298.15 K are used as follows:
H2S H++HS-
H++HS- (5)
(5)
HS- H++S2-
H++S2-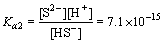 (6)
(6)
It can be deduced from Eqs. (4) to (6) that
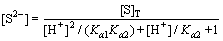 (7)
(7)
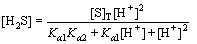 (8)
(8)
The total copper of the solution system can be expressed as follows [28]:
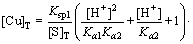
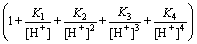 (9)
(9)
Similarly, arsenic in the water has the following chemical equilibrium [29]:
H3AsO3
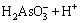
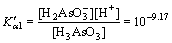 (10)
(10)
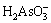

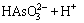
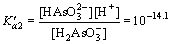 (11)
(11)
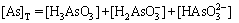 (12)
(12)
It can be deduced from Eqs. (10) to (12) that
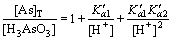 (13)
(13)
Combining Eqs. (3), (8) with Eq. (13), the total arsenic concentration can be expressed as follows:
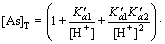
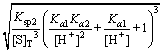 (14)
(14)
For [H+]=10-pH, it can be deduced from Eqs. (9) and (14) that
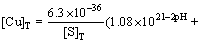
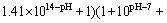
 (15)
(15)

 (16)
(16)
Hypothesizing that CuS and As2S3 stoichio- metrically achieved dissolution equilibrium, then [Cu]T=[S]T, 3[As]T=2[S]T, the lg[Cu]T and lg[As]T as function of pH value are shown in Fig. 2. With the increase of pH value, the solubility of CuS decreases first and then increases. The minimum solubility of CuS is 1.733×10-14 mol/L when the pH value is 7.75. The solubility of As2S3 is greater than that of CuS and increases with the increase of pH value, which favors the possibility of cascade sulfidation of copper and arsenic in the solution.
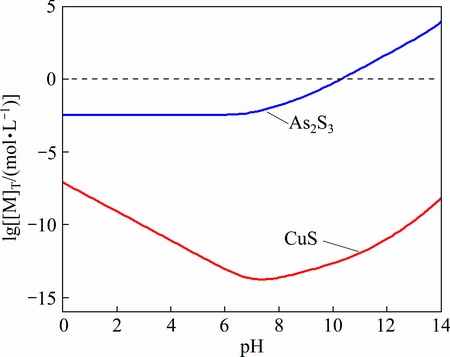
Fig. 2 Solubility equilibrium of CuS and As2S3 as function of pH value
3.2 Effect of parameters on copper and arsenic removal efficiencies
3.2.1 Effect of reaction time
10 mmol and 70 mmol H2S was added into 1000 mL of the simulated solution at 50 °C. The arsenic and copper removal efficiencies at different time are given in Fig. 3.
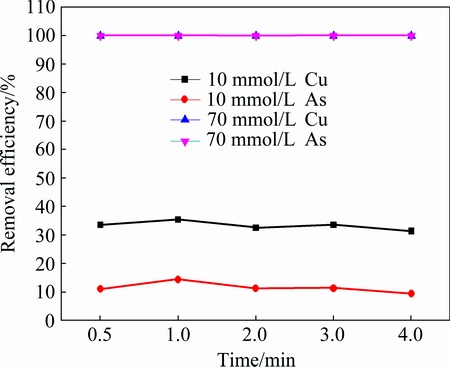
Fig. 3 Arsenic and copper removal efficiencies as function of reaction time
Arsenic and copper removal efficiencies are barely influenced by reaction time, which can be seen from that the arsenic and copper removal efficiencies are constant at 10% and 30% respectively as the reaction time increases from 0.5 to 4 min with addition of 10 mmol sulfide. However, they become 100% when 70 mmol sulfide is added into the solution, which implies that sulfidation of arsenic and copper is finished in 0.5 min.
3.2.2 Effect of reaction temperature
Arsenic and copper removal efficiencies of the reaction system at different temperatures are shown in Fig. 4. The copper removal efficiency increases from 60% to 80% when temperature increases from 24 °C to 60°C, whilst arsenic removal efficiency decreases from 25% to 15%, indicating significant effect of temperature on arsenic and copper removal efficiencies. Moreover, it can be inferred that the improvement of separation efficiency of copper and arsenic can be achieved via raising the temperature. Besides, the temperature of acidic wastewater discharged from the industry process is as high as 55 °C, so the separation of arsenic and copper under high temperature can be realized practically. Therefore, the optimal temperature is chosen as 50 °C.
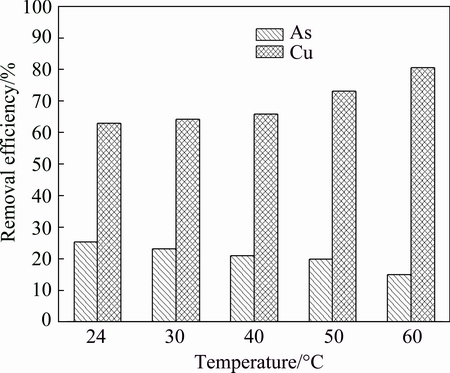
Fig. 4 Arsenic and copper removal efficiencies at different temperatures
3.2.3 Effect of sulfide dosage
Solutions with copper concentration of 600 mg/L and arsenic concentration of 2000 mg/L were prepared to investigate the effect of sulfide dosage on the removal efficiency at temperatures of 30, 40 and 50 °C, respectively. The arsenic and copper removal efficiencies and the residual concentrations are shown in Fig. 5.
Arsenic and copper removal efficiencies increase as the amount of sulfide increases. In all cases, the copper removal efficiency increases more significantly than the arsenic removal efficiency. Both arsenic and copper removal efficiencies are as high as nearly 100% when the addition of sulfide is more than 70 mmol. Comparing the results obtained at 30, 40 and 50 °C, the difference between copper and arsenic removal efficiencies increases with the temperature increasing. The copper removal efficiency is higher than 80% and arsenic removal efficiency is only 20% when certain of sulfide is added into the solution at 50 °C. The results indicate that separation efficiencies of copper and arsenic become higher as the temperature increases, which consists with the results mentioned above.
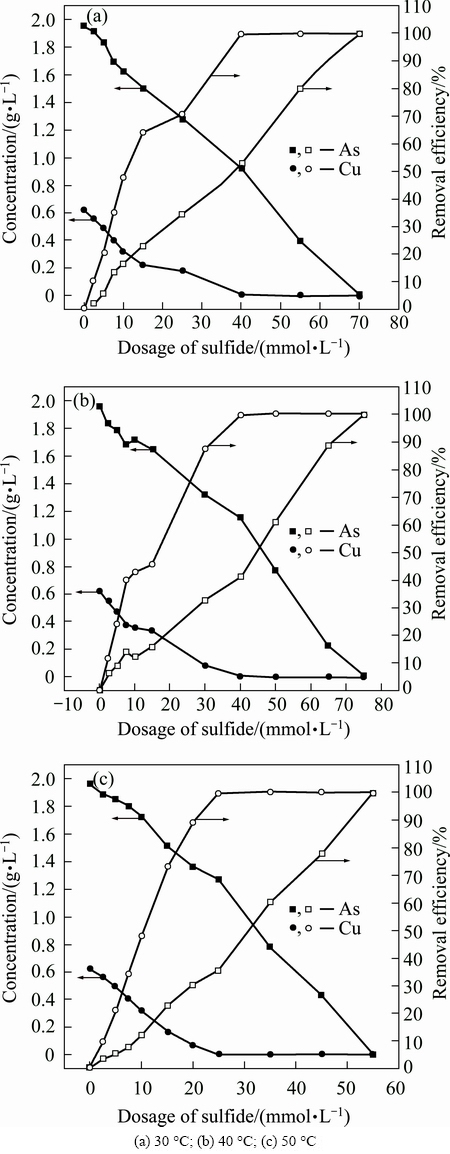
Fig. 5 Arsenic and copper removal efficiencies and concentrations as function of sulifide dosage at different temperatures
3.3 Effect of parameters on replacement of arsenic by copper
3.3.1 Effect of solid-to-liquid ratio
Figure 6 shows arsenic and copper concentrations in acidic wastewater when precipitates generated in the first stage are recycled to the initial solution with different solid-to-liquid ratios. The results show that arsenic concentration in initial solution increases as solid-to- liquid ratio increases, while copper concentration becomes lower under similar conditions. It attributes to the replacement reaction between As2S3 and copper ions, it could be described as follows:
3Cu2++As2S3+4H2O=3CuS+2HAsO2+6H+ (17)
Nearly all of the copper in the initial acidic wastewater is distributed into precipitates and the copper concentration in the initial acidic wastewater is lower than 0.1 mg/L when the solid-to-liquid ratio is more than 10%. Whilst arsenic precipitated into the residue before dissolves into the solution. Therefore, the addition of precipitates generated in the first separation stage into the initial acidic wastewater with certain solid-to-liquid ratio can promote the separation of arsenic and copper, effectively.
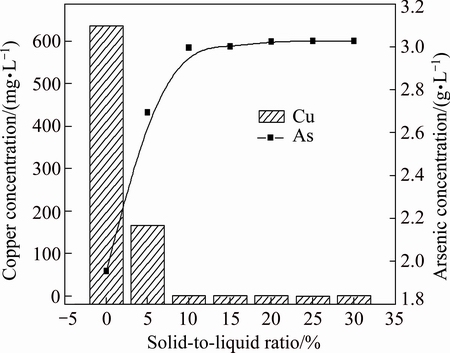
Fig. 6 Arsenic and copper concentrations in acidic wastewater at different solid-to-liquid ratios
3.3.2 Effect of reaction time
Arsenic and copper concentrations in acidic wastewater as a function of reaction time are presented in Fig. 7. The data show that arsenic concentration increases within the first 10 min and then becomes constant at about 3200 mg/L as time increases, while copper concentration decreases rapidly from 110 to 0.5 mg/L within the first 5 min and further decreases to lower than 0.1 mg/L when time is longer than 10 min. The separation efficiencies of arsenic and copper are more than 99%. It is indicated that separation of arsenic and copper is finished within 10 min, which is favorable to the application in the industry.
3.3.3 Effect of temperature
Figure 8 shows arsenic and copper concentrations in acidic wastewater at different temperatures. Secondly, the separation of arsenic and copper is temperature- independent represented by the results that copper concentration in the solution scarcely changes when temperature increases. However, arsenic concentration increases slightly with temperature increasing, which attributes to the re-dissolution of arsenic sulfide in the precipitates at high temperature.
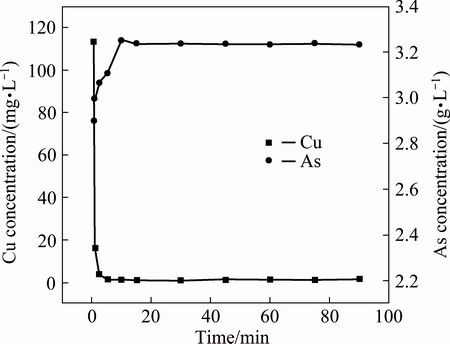
Fig. 7 Arsenic and copper concentrations in acidic wastewater at different reaction time
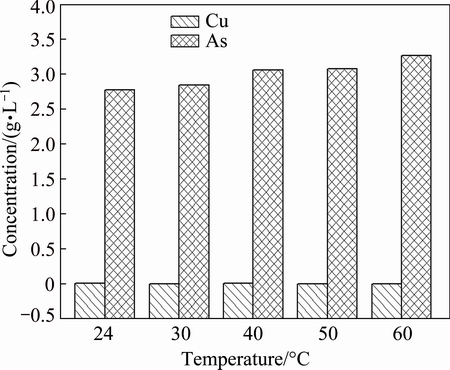
Fig. 8 Arsenic and copper concentrations in acidic wastewater at different temperatures
3.4 Precipitates analysis
SEM-EDS analysis of the precipitate obtained under the optimal condition is shown in Fig. 9. Generally, the morphology of the precipitate exhibits agglomeration of dense, amorphous particles in the micron range. Combined with the results of EDS, the mole ratio of Cu to S is 1.15, which is approximate to 1, indicating that the precipitate mainly consists of CuS. The copper content of precipitate is as high as 63.38% in mass fraction.
4 Conclusions
1) A novel process of treating acidic wastewater containing copper and arsenic is proposed, involving primary separation of copper and arsenic by sulfide precipitation and further separation by the replacement of arsenic in the precipitates by copper in the solution.
2) In the first stage of primary separation, more than 80% copper and nearly 20% arsenic are precipitated with 20 mmol/L H2S at 50 °C within 0.5 min.
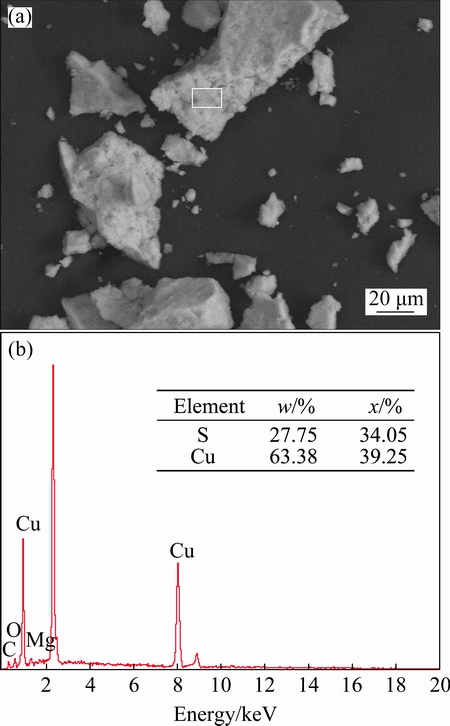
Fig. 9 SEM image (a) and EDS spectrum (b) of precipitate
3) In the second stage of separation, the separation efficiencies of copper and arsenic are more than 99% with precipitates generated in the first stage recycled to the initial solution when solid-to-liquid ratio is not less than 10% at 20 °C within 10 min.
4) Precipitate obtained under the optimal condition mainly consists of CuS. The copper content of precipitate is as high as 63.38% in mass fraction.
References
[1] KAKSONEN A H, RIEKKOLA-VANHANEN M L, PUHAKKA J A. Optimization of metal sulphide precipitation in fluidized-bed treatment of acidic wastewater [J]. Water Research, 2003, 37: 255-266.
[2] MOKONE T P, van HILLE R P, LEWIS A E. Effect of solution chemistry on particle characteristics during metal sulfide precipitation [J]. Journal of Colloid and Interface Science, 2010, 351: 10-18.
[3] FU Feng-lian, XIE Li-ping, TANG Bing, WANG Qi, JIANG Shu-xian. Application of a novel strategy—Advanced Fenton-chemical precipitation to the treatment of strong stability chelated heavy metal containing wastewater [J]. Chemical Engineering Journal, 2012, 189: 283-287.
[4] ADAMCZUK A, KOLODYNSKA D. Equilibrium, thermodynamic and kinetic studies on removal of chromium, copper, zinc and arsenic from aqueous solutions onto fly ash coated by chitosan [J]. Chemical Engineering Journal, 2015, 274: 200-212.
[5] LONG G, PENG Y J, BRADSHAW D. Flotation separation of copper sulphides from arsenic minerals at Rosebery copper concentrator [J]. Minerals Engineering, 2014, 66: 207-214.
[6] PADILLA R, RODRIGUEZ G, RUIZ M C. Copper and arsenic dissolution from chalcopyrite-enargite concentrate by sulfidation and pressure leaching in H2SO4-O2 [J]. Hydrometallurgy, 2010, 100(3): 152-156.
[7] WANG Ting, YANG Wei-chun, SONG Ting-ting, LI Chao-fang, ZHANG Li-yuan, WANG Hai-ying, CHAI Li-yuan. Cu doped Fe3O4 magnetic adsorbent for arsenic: Synthesis, property, and sorption application [J]. RSC Advances, 2015, 5(62): 50011-50018.
[8] WANG Zhi-feng, CUI Zhao-jie, LIU Lei, MA Qian-chi, XU Xiao-ming. Toxicological and biochemical responses of the earthworm eisenia fetida exposed to contaminated soil: Effects of arsenic species [J]. Chemosphere, 2016, 154: 161-170.
[9] HANSEN H K, GUTIERREZ C, FERREIRO J, ROJO A. Batch electrodialytic treatment of copper smelter wastewater [J]. Minerals Engineering, 2015, 74: 60-63.
[10] MONSER L, ADHOUM N. Modified activated carbon for the removal of copper, zinc, chromium and cyanide from wastewater [J]. Separation and Purification Technology, 2002, 26: 137-146.
[11] FILIPPOU D, ST-GERMAIN P, GRAMMATIKOPOULOS T. Recovery of metal values from copper-arsenic minerals and other related resources [J]. Mineral Processing and Extractive Metallurgy Review, 2007, 28(4): 247-298.
[12] GUTIERREZ C, HANSEN H K, NUNEZ P, VALDES E. Electrochemical peroxidation using iron nanoparticles to remove arsenic from copper smelter wastewater [J]. Electrochimica Acta, 2015, 181: 228-232.
[13] YAN Xu, LI Qing-zhu, CHAI Li-yuan, YANG Ben-tao, WANG Qing-wei. Formation of abiological granular sludge – A facile and bioinspired proposal for improving sludge settling performance during heavy metal wastewater treatment [J]. Chemosphere, 2014, 113: 36-41.
[14] WANG Ting, ZHANG Li-yuan, LI Chao-fang, YANG Wei-chun, SONG Ting-ting, TANG Chong-jian, MENG Yun, DAI Shuo, WANG Hai-ying, CHAI Li-yuan, LUO Jian. Synthesis of core-shell magnetic Fe3O4@poly(m-phenylenediamine) particles for chromium reduction and adsorption [J]. Environmental Science & Technology, 2015, 49(9): 5654-5662.
[15] CHAI Li-yuan, WANG Qing-wei, LI Qing-zhu, YANG Zhi-hui, WANG Yun-yan. Enhanced removal of Hg(II) from acidic aqueous solution using thiol-functionalized biomass [J]. Water Science and Technology, 2010, 62(9): 2157-2165.
[16] WANG Qing-wei, QIN Wen-qing, CHAI Li-yuan, LI Qing-zhu. Understanding the formation of colloidal mercury in acidic wastewater with high concentration of chloride ions by electrocapillary curves [J]. Enviromental Science and Pollution Research, 2014, 21: 3866-3872.
[17] ZENG J X, YE H Q, HUANG N D, LIU J F. Selective separation of Hg(II) and Cd(II) from aqueous solutions by complexation- ultrafiltration process [J]. Chemosphere, 2009, 76(5): 706-710.
[18] YANG W C, ZHAO N, ZHANG N, CHEN W, KAN A T, TOMSON M B. Time-dependent adsorption and resistant desorption of arsenic on magnetite nanoparticles: Kinetics and modeling [J]. Desalination and Water Treatment, 2012, 44: 100-109.
[19] MOHAMMED A, AKRAM D, CHEDLY T, NIDAL H. Combined humic substance and heavy metals coagulation, and membrane filtration under saline conditions [J]. Desalination, 2010, 253: 46-50.
[20] MISRA R K, JAIN S K, KHATRI P K, Iminodiacetic acid functionalized cation exchange resin for adsorptive removal of Cr(VI), Cd(II), Ni(II) and Pb(II) from their aqueous solutions [J]. Journal of Hazardous Materials, 2011, 185: 1508-1512.
[21] WANG Yang-yang, PENG Bing, YANG Zhi-hui, TANG Chong-jian, CHEN Yue-hui, LIAO Qi, LIAO Ying-ping. Treatment of Cr(VI) contaminated water with Pannonibacter phragmitetus BB [J]. Environmental Earth Sciences, 2014, 71(10): 4333-4339.
[22] STALIDIS G A, MATIS K A, LAZARIDIS N K. Selective separation of Cu, Zn, and As from solution by flotation techniques [J]. Separation Science and Technology, 1989, 24(1-2): 97-109.
[23] ALISON E L. Review of metal sulphide precipitation [J]. Hydrometallurgy, 2010, 104: 222-234.
[24] ZOU Lian-hua, XUE Yu-lan. Sulfide precipitation flotation for treatment of acidic mine waste water [J]. Transactions of Nonferrous Metals Society of China, 2000, 10: 106-109.
[25] HUISMAN J L, SCHOUTEN G, SCHULTZ C. Biologically produced sulphide for purification of process streams, effluent treatment and recovery of metals in the metal and mining industry [J]. Hydrometallurgy, 2006, 83(1): 106-113.
[26] BHATTACHARYYA D, JUMAWAN A B Jr, GRIEVES R B. Separation of toxic heavy metals by sulfide precipitation [J]. Separation Science and Technology, 1979, 14(5): 441-452.
[27] VEEKEN A H M, de VRIES S, van DER MARK A, RULKENS W H. Selective precipitation of heavy metals as controlled by a sulfide-selective electrode [J]. Separation Science and Technology, 2003, 38(1): 1-19.
[28] CHAI Li-yuan, WANG Hai-tang, YOU Xiang-yu, WANG Qing-wei, SHU Yu-de. Thermodynamics equilibrium of M(II)-S-H2O system [J]. Nonferrous Metals Science and Engineering, 2012, 3(5): 8-13. (in Chinese)
[29] HELZ G R, TOSSELL J A. Thermodynamic model for arsenic speciation in sulfidic waters: A novel use of ab initio computations [J]. Geochimica et Cosmochimica Acta, 2008, 72(18): 4457-4468.
污酸废水中铜和砷的气-液梯级硫化与分离
蒋国民1,3,彭 兵1,2,柴立元1,2,王庆伟1,2,史美清2,王云燕1,2,刘 恢1,2
1. 中南大学 冶金与环境学院,长沙 410083;2. 中南大学 国家重金属污染防治工程技术研究中心,长沙 410083;
3. 长沙赛恩斯环保科技有限公司,长沙 410000
摘 要:通过气-液硫化反应初步分离污酸废水中的铜和砷,并将一级铜、砷分离渣按一定比例加入污酸原液中实现溶液中铜和渣中砷的置换分离。考察反应时间、温度和H2S用量对铜、砷去除率的影响,同时考察固液比、时间和温度对铜、砷置换反应的影响。当在50 °C时加入20 mmol/L的H2S时,在0.5 min时间内即可使铜的去除率达到80%以上,且砷的去除率约为20%。将铜、砷一级分离渣按照10%以上的固液比例加入污酸废水中,在20 °C下10 min后由于铜、砷置换作用,废酸中铜和砷分离率达到99%以上。沉渣中主要物相为CuS,富集的铜渣中铜的质量分数为63.38%。
关键词:污酸废水;铜;砷;梯级硫化;分离
(Edited by Wei-ping CHEN)
Foundation item: Projects (51304251, 51504299) supported by the National Natural Science Foundation of China; Project (201509050) supported by Special Program on Environmental Protection for Public Welfare, China; Project (k1502037-31) supported by Key Project of Changsha, China
Corresponding author: Qing-wei WANG; Tel: +86-731-88830577; Fax: +86-731-88710171; E-mail: qw_wang@csu.edu.cn
DOI: 10.1016/S1003-6326(17)60107-9
Abstract: Copper and arsenic in acidic wastewater were separated by cascade sulfidation followed by replacement of arsenic in the precipitates by copper in the solution which was realized by recycling precipitates obtained in the first stage into the initial solution. The effects of reaction time, temperature and H2S dosage on copper and arsenic removal efficiencies as well as the effects of solid-to- liquid ratio, time and temperature on the replacement of arsenic by copper were investigated. With 20 mmol/L H2S at 50 °C within 0.5 min, more than 80% copper and nearly 20% arsenic were precipitated. The separation efficiencies of copper and arsenic were higher than 99% by the replacement reaction between arsenic and copper ions when solid-to-liquid ratio was more than 10% at 20 °C within 10 min. CuS was the main phases in precipitate in which copper content was 63.38% in mass fraction.


