
Corrosion and electrochemical behavior of AZ31D
magnesium alloys in sodium chloride
SHAN Da-yong(单大勇)1 , ZHOU Wan-qiu (周婉秋)1,2, HAN En-hou(韩恩厚)1 , KE Wei(柯 伟)1
1. Institute of Metal Research, Chinese Academy of Sciences, Shenyang 110016, China;
2. College of Material Science and Engineering, Beijing University of Chemical Technology, Beijing100029, China
Received 28 July 2006; accepted 15 September 2006
Abstract:
The corrosion and electrochemical behavior of extruded AZ31D magnesium alloys in NaCl solution were investigated using SEM, XRD and electrochemical method. It is found that AZ31D is susceptive to Cl- ion, and the open circuit potential shifts to more negative values with increasing chloride concentration. Pitting occurs at corrosion potential and corrosion area enlarges with enhanced polarization. Tafel slopes of the cathode branches in different testing solution are almost the same. Cl- concentration affects cathode course slightly. High frequency capacitive loops shrink with the increase of Cl- concentration. Corrosion initiates from the grain boundary and spreads to entire surface with time.
Key words:
AZ31D magnesium alloys; corrosion behavior; chloride ion;
1 Introduction
Magnesium alloys were developed primarily for transportation application that has obvious need for light mass material[1]. Poor corrosion resistance has long been a recognized property of magnesium, and this property has played a prominent role in preventing more wide spread use of magnesium alloys[2-3]. The corrosion behavior depends considerably on the microstructure and the environment to which it is exposed. The microstructure varies with the method of processing which gives rise to different corrosion behavior. Considerable studies on the effect of microstructure on the corrosion and electrochemical behavior of cast magnesium alloys, especially on die cast and ingot AZ91 alloys, have been conducted[4-7]. It was considered that the second phase Mg17Al12 plays an important role in corrosion of magnesium alloys. AZ31 magnesium alloy is a single solid solution, and no β phase precipitates along grain boundary. However, few researches were carried out on the corrosion behavior of AZ31D magnesium alloys. The effect of microstructure on the corrosion of AZ31 has seldom been reported. MAKAR et al[8] and SONG et al [3] pointed out that grain boundary always acts as cathode opposite to inner grain during corrosion of magnesium alloys. In this work, the corrosion behavior of extruded AZ31D magnesium alloy is investigated in aqueous environment containing chloride ion.
2 Experimental
The extruded AZ31D plates were used with the composition of 2.5%-3.5% Al, 0.6%-1.4% Zn, 0.2%- 1.0% Mn, <0.01% Si, <0.01% Cu, <0.001% Ni, <0.004% Fe, and balance Mg. The samples for electrochemical measurement were molded into epoxy resin with only one side exposed as working surface available. The working surface was polished with grit SiC paper from 800# to 1 200#, washed with distilled water and dried by warm flowing air. The electrochemical tests were carried out using EG &G M273 potentiostat. A saturated calomel electrode was used as reference electrode, and platinum flake was used as auxiliary electrode. Polarization curve was measured at a scan rate of 0.5 mV/s. EIS measurements were conducted using EG &G M273 potentiostat coupled with M5210 lock-in amplifier with a perturbing signal of AC amplitude of 5 mV and a frequency ranging from 100 kHz to 5 mHz. The testing solution was prepared with AR grade NaCl in distilled water and saturated with Mg(OH)2 to keep pH value at 11 during the measurement. Morphology was observed by SEM. The microstructure was tested by XRD. The chemical composition was analyzed by EDX.
3 Results and discussion
3.1 Microstructure
AZ31D is single phase α with some AlMn particle scattered on metal matrix as illustrated in Fig.1. The conclusion concerning the phase composition was confirmed by X-ray diffraction pattern. The XRD pattern contains only peaks corresponding to α(Fig.2).
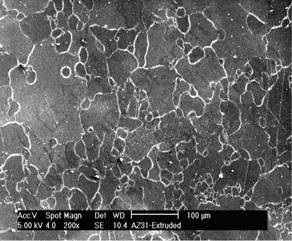
Fig.1 Microstructure of extruded AZ31D
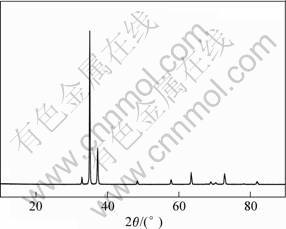
Fig.2 XRD pattern of AZ31D
3.2 Electrochemical measurement
Fig.3 reveals the variation of open circuit potential (OCP) for extruded AZ31D in different NaCl concentration solutions. The electrode potential shifts positively with immersion time increasing and reaches a steady value after 30 min immersion. And potential decreases with NaCl concentration increasing. The potentiodynamic polarization curves for extruded AZ31D magnesium alloys in different NaCl concentrations are presented in Fig.4. The polarization curves all reach pitting potential at φcorr about -1.60 V(vs SCE) for the four NaCl solutions and φcorr reduces with the increase of Cl- concentration. Tafel slopes for the cathode branches of polarization curves are almost the same. Cl- concentration affects cathode process slightly. Metal surface keeps in brightness and no attack happens during the cathodic polarization. Pitting occurs immediately when potential arrives at φcorr on the surfaces of all the four samples in different NaCl solutions.
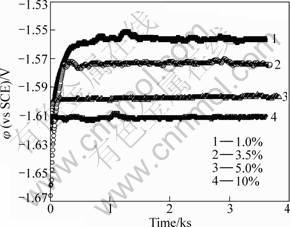
Fig.3 φocp vs time curves
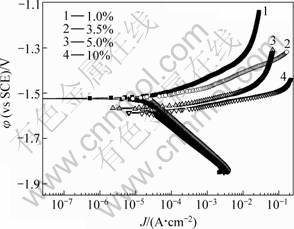
Fig.4 Polarization curves of AZ31D
Corrosion initiates from a few small pitting and enlarges gradually and new pitting occurs subsequently. Pitting occurrence and spreading rate in dilute NaCl solution are lower than in dense liquid. Most of the sample surface is not destroyed in low concentration NaCl solution after polarization tests and the original morphology can still be observed. Samples in 5% and 10% NaCl solutions are seriously corroded after the test. In the pitting position, the content of magnesium is low and the contents of aluminum and chlorine are relatively high. Corrosive Cl- ion promotes the corrosion of metal and magnesium dissolves preferentially. The EDX spectrum of pitting is revealed in Fig.5. The EIS in different NaCl concentrations for extruded AZ31D is presented in Fig.6. High frequency capacitive loops shrink with the increase of Cl- concentration. There are a large capacitive loop in high frequency and a small capacitive loop in low frequency in 1% NaCl solution. With Cl- ion concentration increasing, the inductive loop appears in very low frequency at OCP. The appearance of inductive loop is related to the pitting of alloys at OCP. Pitting attack can be observed in EIS testing process and hydrogen separates out at pitting position. HF capacitive loop is related to the capacitance of double layers in the interface of metal/solution and electrochemical reaction resistance, and LF capacitive loop involves in the evolution of hydrogen.
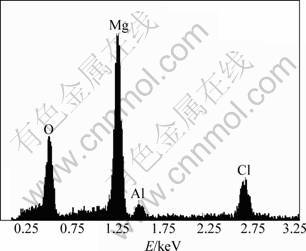
Fig.5 EDX spectrum of pitting site
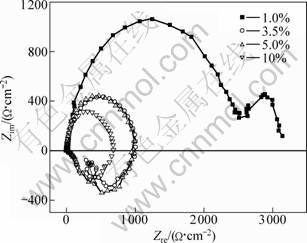
Fig.6 EIS of AZ31D in NaCl solution
3.3 Corrosion morphology
Fig.7 illustrates the typical surface feature of AZ31 after a few minutes exposure. It can be clearly seen that corrosion starts initially at localized sites, preferentially from grain boundary rather than at the grain interior. Aggressive medium causes attack at grain boundary preferentially and cracking appears. However, around the dispersed AlMn particle, no obvious corrosion can be observed. The microstructure of metal matrix influences pitting remarkably, and pitting is prone to take place on pitting sensitive position. When AZ31D is immersed in a solution containing Cl-, Cl- absorbs preferentially on the pitting sensitive position such as grain boundary. High chemical activity of grain boundary causes the corrosion to start from grain boundary, and the localized attack invades the entire surface with continued exposure to give a general corrosion pattern with corrosion product remained on the surface as illustrated in Fig.8. The corrosion product congregates on metal surface which offers protection for metal substrate at some extent.
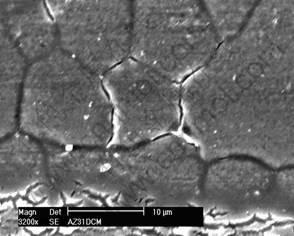
Fig.7 Morphology of initial attack

Fig.8 Morphology after 96 h immersion
4 Conclusions
1) The extruded AZ31D alloy is single phase α solid solution with dispersed AlMn particle on metal matrix.
2) Corrosion initiates from the grain boundary and spreads to entire surface with exposure time increasing.
3) The corrosion rate increases with Cl- concentration increasing, pitting occurs at corrosion potential, and Cl- concentration does not affect cathode process. Corrosive Cl- ion promotes the corrosion and magnesium dissolves preferentially.
References
[1] ZENG Rong-chang, KE Wei, XU Yong-bo, HAN En-hou, ZHU Zi-rong. Recent development and application of magnesium alloys[J]. Acta Metallurgica Sinica, 2001, 37 (7): 673-685.(in Chinese)
[2] SONG G, STJOHN D H. Corrosion of magnesium alloys in commercial engine coolants[J]. Material and Corrosion, 2005, 56(1): 15-23.
[3] SONG Guang-ling, ATRENS A. Corrosion mechanisms of magnesium alloys[J]. Advanced Engineering Materials, 1999(1): 11-33.
[4] AMBAT R, AUNG N N, ZHOU W. Evaluation of microstructure effect on corrosion behavior of AZ91D magnesium alloy[J]. Corrosion Science, 2000, 42: 1433-1455.
[5] SONG Guang-ling, ATRENS A, WU Xian-liang. Corrosion behavior of AZ21, AZ501 and AZ91 in sodium chloride[J]. Corrosion Science, 1998, 40(10): 1769-1791.
[6] LUNDER O, LEIN J E, KR AUNE T, NISANCIOGLU K. The role of Mg17Al12 phase in the corrosion of Mg alloys AZ91[J]. Corrosion, 1989, 45(9): 741-748.
[7] BELDJOUDI T, FIAUD C, ROBBIOLA L. Influence of homogenization and artificial aging heat treatments on corrosion behavior of Mg-Al alloys[J]. Corrosion Science, 1993, 49(9): 738-745.
[8] MARKAR G L, KRUGER J. Corrosion of magnesium[J]. International Materials Reviews, 1993, 38(3): 138-153.
(Edited by YUAN Sai-qian)
Foundation item: Project (2001AA331050) supported by the Hi-tech Research and Development Program of China
Corresponding author: ZHOU Wan-qiu; Tel: +86-24-23893115; E-mail: wqzhou@imr.ac.cn


