Trans. Nonferrous Met. Soc. China 26(2016) 1688-1695
Leaching kinetics of zinc silicate in ammonium chloride solution
Sheng-hai YANG, Hao LI, Yan-wei SUN, Yong-ming CHEN, Chao-bo TANG, Jing HE
School of Metallurgy and Environment, Central South University, Changsha 410083, China
Received 26 August 2015; accepted 12 April 2016
Abstract:
The leaching kinetics of zinc silicate in ammonium chloride solution was investigated. The effects of stirring speed (150-400 r/min), leaching temperature (95-108 °C), particle size of zinc silicate (61-150 μm) and the concentration of ammonium chloride (3.5-5.5 mol/L) on leaching rate of zinc were studied. The results show that decreasing the particle size of zinc silicate and increasing the leaching temperature and concentration of ammonium chloride can obviously enhance the leaching rate of zinc. Among the kinetic models of the porous solids tested, the grain model with porous diffusion control can well describe the zinc leaching kinetics. The apparent activation energy of the leaching reaction is 161.26 kJ/mol and the reaction order with respect to ammonium chloride is 3.5.
Key words:
zinc silicate; ammonium chloride; leaching; kinetics; porous diffusion control;
1 Introduction
With the depletion of world supplies of high grade zinc sulfides ores, more and more attention will be focused on the extraction of zinc from the zinc oxide ores. Zinc oxide ores are generally found in the forms of carbonate and silicate such as smithsonite (ZnCO3), hydrozincite (2ZnCO3·3Zn(OH)2), zincite (ZnO), willemite (Zn2SiO4) and hemimorphite (Zn4Si2O7(OH)2·H2O) in the world [1,2]. Within the foreseeable future, the annual production of zinc from non-sulfide sources will exceed 10% of global zinc production. Currently, the leaching agents, such as sulfuric acid [3-5] and ammoniacal solutions [6,7], have been used to extract zinc from zinc oxide ores. Calcium/magnesium carbonate gangues containing in zinc oxide ores cause tremendous increase in acid consumption and gypsum harden in acid-leaching process, while ammonia is an attractive reagent for its no reaction with carbonate gangues, low inventory cost and amenability to regeneration [7]. Ammonia is commonly used in the extraction industry of cobalt, nickel and copper due to the formation of stable metal ammine complexes, leading to higher solubility in most cases. Other harmful impurities such as Fe, Pb, Ca and Mg precipitate as oxides because of their poor complexation ability with ammonia [8].
The leaching kinetics of zinc silicate is far less studied than that of zinc sulfides and zinc carbonates ores. In acid leaching, TERRY and MONHEMIUS [4] studied the leaching kinetics of both natural and synthetic willemite (Zn2SiO4) and hemimorphite. They found that the acidic dissolution was controlled by diffusion for hemimorphite and primarily controlled by chemical and diffusion step for willemite. ABDEL- AAL [9] studied the leaching kinetics of low grade zinc silicate, and proposed that the process was controlled by diffusion on an “ash” layer and the apparent activation energy was calculated as 13.4 kJ/mol. In alkaline leaching, SANTOS et al [10] studied the leaching kinetics parameters of zinc silicate ores in sodium hydroxide solutions. They found that the leaching process was a progressive reduction in particle size controlled by chemical step with an apparent activation energy of 67.8 kJ/mol, which was in good agreement with the results of CHEN et al [11]. In ammoniacal solution, DING et al [8] found that the Elovich equation typified the dissolution behavior of hemimorphite in ammoniacal solution with an activation energy of 57.6 kJ/mol which was characteristic for a chemically controlled process.
The most significant characteristics of leaching in an ammonium chloride solution is the practically constant and almost neutral pH, generally in the 6-7 range. This makes the calcium, magnesium, silicon, iron and other minor elements (such as As, Sb, Bi) containing in the minerals remain in the residues during the leaching process [12]. Zinc could be extracted by purification or solvent extraction and electrowinning [12-14]. As the leaching kinetics of zinc silicate in ammonium chloride solutions has not been well studied, the purpose of the present work is to assess the leaching kinetic parameters of the zinc silicate in the ammonium chloride solutions, and it will be a guidance for leaching zinc silicate using ammonium chloride solutions.
2 Experimental
2.1 Materials
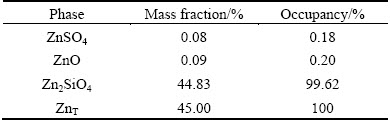
Zinc silicate was synthesized according to Ref. [15]. The X-ray diffraction (XRD) revealed that the sample produced was zinc silicate (Fig. 1). The mineralogical composition is presented in Table 1. Analytical grade of ammonium chloride and deionized water were used in the experiments.
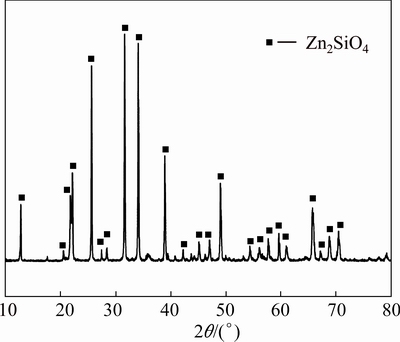
Fig. 1 XRD pattern of zinc silicate
Table 1 Mineralogical composition of zinc silicate
2.2 Leaching methods
The leaching solutions were prepared from reagent-grade ammonium chloride, dissolved in deionized water, according to the desired ammonium chloride concentration (3.5-5.5 mol/L). All experiments were carried out by using zinc silicate particles with 45-61 μm in size, except in experiments where effect of particle size on zinc extraction was investigated. The leaching temperature was in the range of 95-108 °C and the stirring speed was in the range of 150-400 r/min.
The leaching process was carried out in a flat-bottomed spilt flask (2000 mL total volume), with a three-necked top, equipped with a mercury thermometer, a delivery tube for the leaching solutions and a condenser tube which decreased the evaporation loss of the solution. Temperature control of the flask contents within ±0.5 °C was achieved by a thermostat oil bath cauldron. Agitation was provided by a magnetic stirrer that enabled adequate dispersion of the mineral particles. The solution volume was 1000 mL and the solid concentration was 5 g/L. At selected time intervals, a known amount (5 mL) of slurry was withdrawn by sampling port and filtered quickly. 0.5 mL of filtrate was then diluted as 250 mL for zinc analysis using an atomic absorption spectrometer (TAS-990).
The leaching rate of zinc, R, is defined by
R=cV/(mω) (1)
where c, V, m and ω are the zinc concentration in filtration, the volume (we assumed it constant), mass and zinc content of the added material, respectively.
The zinc silicate and leaching residues were analyzed by XRD (Japan Rigaku Model TTRIII + 40 kV/250 mA with Cu Kα radiation), SEM and backscattered electron micrographs (BEM) (Japan Jeol JSM-6360LV).
3 Results
3.1 Effect of stirring speed on leaching rate of zinc
To investigate the effect of stirring speed on the leaching rate, experiments were carried out at five different stirring speeds in the range of 150-400 r/min. The results, as presented in Fig. 2, show little effect of stirring speed on zinc extraction. Therefore, the leaching process does not seem to be controlled by mass transfer through the liquid boundary layer. As a result, the stirring speed was kept at 350 r/min, unless otherwise stated in this work.
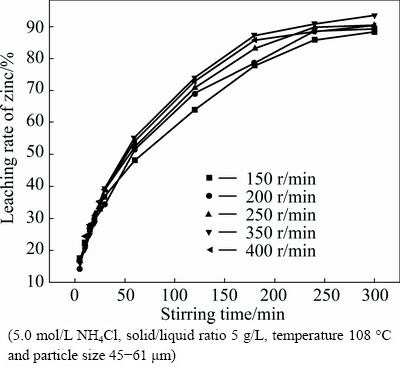
Fig. 2 Effect of stirring speed on leaching rate of zinc
3.2 Effect of leaching temperature on leaching rate of zinc
The effect of leaching temperature from 95 to 108 °C on the leaching rate of zinc was studied. The results, as presented in Fig. 3, show that the leaching rate of zinc increases significantly with the increase of temperature. After 300 min, the leaching rate of zinc increases from 50% to 93% as the temperature increases from 105 to 108 °C. Figure 4 shows the XRD patterns of the leaching residues obtained at different leaching temperatures. The major phase of leaching residue is silica dioxide when the leaching temperature is higher than 108 °C.
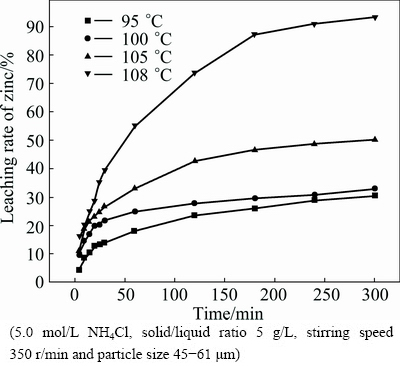
Fig. 3 Effect of leaching temperature on leaching rate of zinc
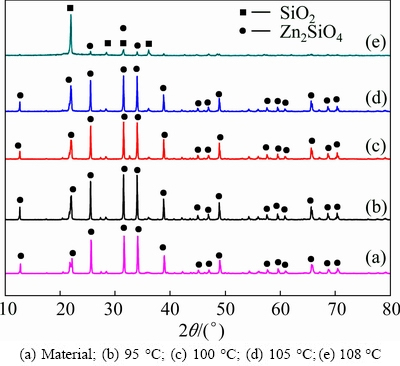
Fig. 4 XRD patterns of leaching residues at different leaching temperatures
3.3 Effect of particle size on leaching rate of zinc
The influence of particle size from 45-61 to 98-150 μm on the leaching rate of zinc was investigated. The results, as presented in Fig. 5, show that decreasing particle size enhances zinc extraction. As expected, the smaller the size of the particles, the faster the leaching rate. It seems that decreasing the particle size can improve the reaction area.
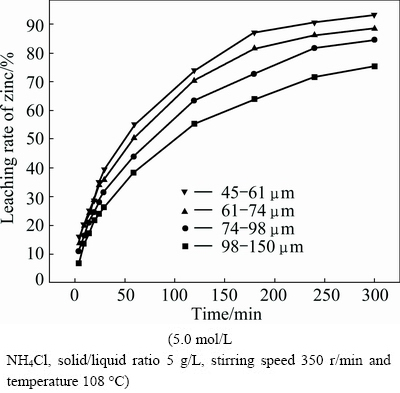
Fig. 5 Effect of particle size on leaching rate of zinc
3.4 Effect of ammonium chloride concentration on leaching rate of zinc
The effect of ammonium chloride concentration on the leaching rate of zinc was studied in the range of 3.5 to 5.5 mol/L. The results, as presented in Fig. 6, show that the leaching rate of zinc increases significantly with increasing the ammonium chloride concentration. About 94% of zinc is extracted in 5.5 mol/L ammonium chloride solution after 300 min.
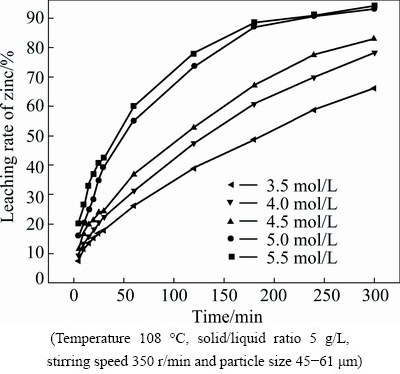
Fig. 6 Effect of ammonium chloride concentration on leaching rate of zinc
3.5 Characterization of leaching residues
The zinc silicate samples before and after leaching were characterized by SEM (see Fig. 7) and BEM (see Fig. 8). The solid particles present a rough and porous surface, as shown in Fig. 7(a). The micrographs of the leaching residues exhibit a further increase in the roughness and porosity of the solid. For instance, comparing Figs. 7(c) and (d) with (a) reveals the occurrence of more holes on the surface of the solid particles. The BEM images (Fig. 8) of the polished section of the zinc silicate before and after leaching exhibit that the reticulate structure appears but no reaction product layer occurs on the particle surface. Figure 9 shows the XRD patterns of the leaching residues at different reaction time. Therefore, it is deduced that the zinc leaching process might be controlled by diffusion of the reagent in the porous structure of the particles, as observed by GEORGIOU and PAPANGELAKIS [16] in pressure leaching experiments carried out with limonitic laterite ores.
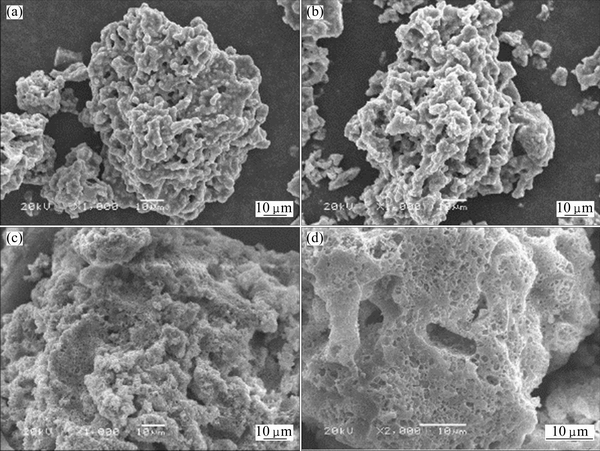
Fig. 7 SEM images of zinc silicate before leaching (a), after 30% leaching rate of zinc (b), after 75% leaching rate of zinc (c), and after 93% leaching rate of zinc (d)
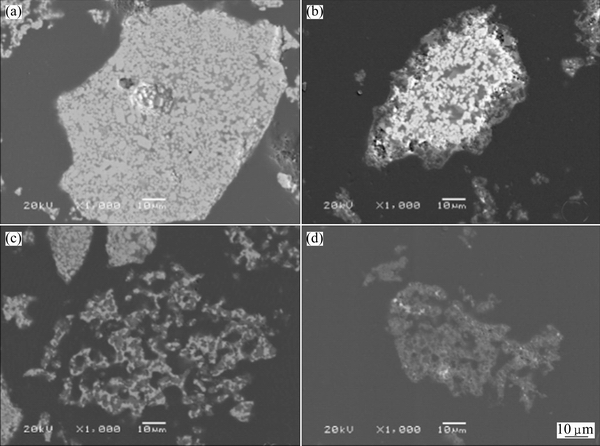
Fig. 8 BEM images of zinc silicate (polished section) before leaching (a), after 30% leaching rate of zinc (b), after 75% leaching rate of zinc (c) and after 93% leaching rate of zinc (d)
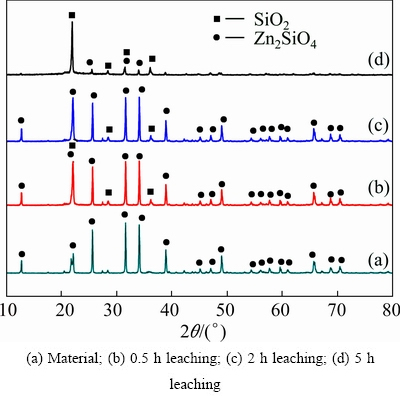
Fig. 9 XRD patterns of leaching residues at different leaching time
4 Kinetic discussion
The comparison of the SEM (Fig. 7) and BEM images (Fig. 8) of the zinc silicate and the leaching residues shows that the residues become very porous with an increase in leaching rate of zinc. This suggests that the leaching process can be described according to the shrinking core model. The reaction rate might be controlled by chemical reaction on the particle surface, diffusion through the inert particle pore, diffusion through liquid film on the surface of the particle, or a combination of two or three of these processes [17].
When the surface chemical process alone is the rate limitation step, the following expression of the shrinking core model can be used to describe the leaching kinetics of the process:
1-(1-R)1/3=kRt, kR=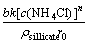 (2)
(2)
where kR is the reaction rate constant; t is time; R is the reacted fraction of zinc; b is the stoichiometric coefficient; k is the chemical rate constant; c(NH4Cl) is the concentration of ammonium chloride; n is the reaction order; ρsillicate is the silicate molar density; and r0 is the initial particle radius.
Similarly, when the diffusion of the reagent through a product layer is the rate-controlling step, the following expression of the shrinking core model is achieved to describe the dissolution kinetics:
1-3(1-R)2/3+2(1-R)=kdt, kd=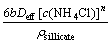 (3)
(3)
where kd is the apparent diffusion rate constant for nonporous particles; and Deff is the effective diffusion coefficient.
Figure 10 presents the fitting curves of the shrinking core model with chemical reaction control and product diffusion control to the experimental data. It shows that the model for product diffusion control is better than that for chemical reaction control. However, the dissolution of the zinc silicate occurs without the formation of a product layer, as shown in Figs. 7 and 8. Therefore, this model is not consistent with the SEM and BEM analysis of the leaching residues. Thus, a different model, which takes into account the effect of porosity, should be built so that the zinc silicate leaching can be adequately described.

Fig. 10 Fitting shrinking core models with chemical reaction control and product diffusion control (conditions as shown in Fig. 3)
Several models have been proposed to describe the leaching kinetics of porous solids, such as the random pore model, the uniform pore model and the grain model. The random pore model could not be applied to representing the leaching process as observed elsewhere [18]. The uniform pore model with chemical control was also tested to describe the leaching kinetics, but did not produce a good fit to the experimental data, as observed in other hydrometallurgical systems [18,19]. The grain pore model will be discussed below [5].
The grain model can also be applied to describing the leaching kinetics of porous solids. The model gives the following expression for spherical particles:
1-3(1-R)2/3+2(1-R)=kDt, kD=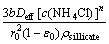 (4)
(4)
where kD is the reaction rate constant, and ε0 is the initial porosity of the solid. The plot of 1-3(1-R)2/3+2(1-R) versus leaching time, t, at different temperatures, is given in Fig. 11. As shown in Fig. 11, a good fit is observed when 1-3(1-R)2/3+2(1-R) is applied to the experimental data. Figure 12 shows the Arrhenius plot constructed with the reaction rate constant value, kD, calculated from the data presented in Fig. 11. The calculated apparent activation energy determined for the pore diffusion control is 161.26 kJ/mol.
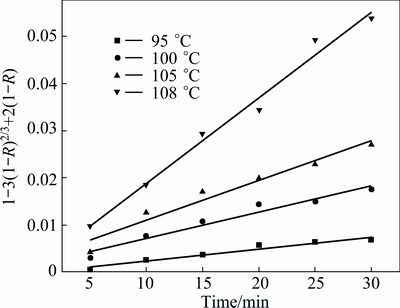
Fig. 11 Plot of 1-3(1-R)2/3+2(1-R) vs t at different temperatures
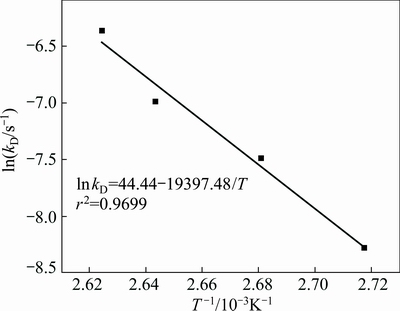
Fig. 12 Arrhenius plot for leaching of zinc silicate
The dissolution of zinc occurs as a consequence of the formation of ternary complexes in neutral ammonium chloride solutions with the general formula of 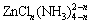 [20]. The ion radius of ternary complexes is larger than that of single ion Zn2+, which results in the lower diffusion speed, thus the higher apparent activation energy of the pore diffusion control is obtained than that in other leaching media (Table 2).
[20]. The ion radius of ternary complexes is larger than that of single ion Zn2+, which results in the lower diffusion speed, thus the higher apparent activation energy of the pore diffusion control is obtained than that in other leaching media (Table 2).
According to the data of Fig. 5, the plot of Eq. (4) vs leaching time, t, for different particle sizes is given in Fig. 13. Clearly, the model also fits well. Furthermore, from the analysis of Eq. (4), there is a clear dependence of the reaction rate constant, kD, with the inverse square of initial particle radius (1/r2 0).
Table 2 Selected values of activation energies reported for leaching of zinc silicates
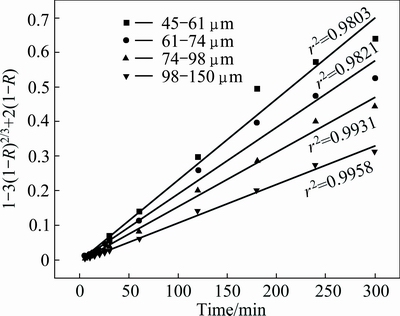
Fig. 13 Plot of 1-3(1-R)2/3+2(1-R) vs t for different particle sizes
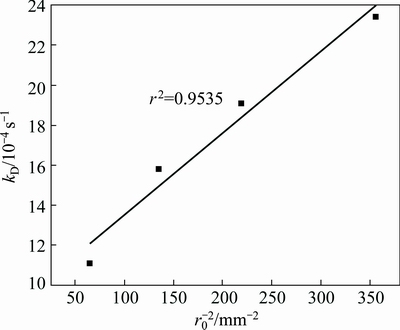
Fig. 14 Plot of kD vs 1/r2 0
Figure 14 shows the plot of kD vs 1/r2 0 that is obtained from the linear fitting of the data in Fig. 5 into Eq. (4). As shown in Fig. 14, a linear relationship between the rate constant and the square of the inverse of the initial particle radius, 1/r2 0, is obtained. This figure might support the application of the grain model with pore diffusion control to describe the dissolution kinetics of the zinc silicate.
According to the data in Fig. 6, the plot of Eq. (4) vs leaching time, t, at different ammonium chloride concentrations is given in Fig. 15, which is in good agreement with the grain model. Using the fitted kD values, plot of ln kD vs ln[c(NH4Cl)] at different ammonium chloride concentrations is given in Fig. 16. The slope of the regressive line is 3.5, which is equal to the reaction order of ammonium chloride.
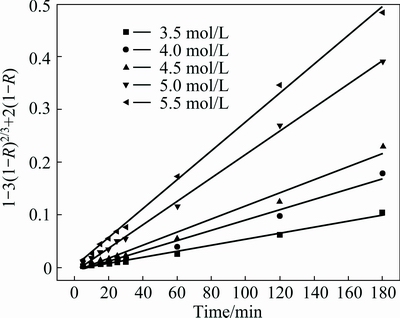
Fig. 15 Plot of 1-3(1-R)2/3+2(1-R) vs t at different ammonium chloride concentrations
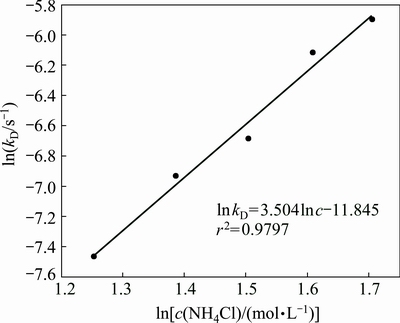
Fig. 16 Plot of ln kD vs ln[c(NH4CL)]
5 Conclusions
1) The leaching kinetics of synthesized zinc silicate in ammonium chloride solutions was studied. The results reveal that the leaching rate of zinc is enhanced slightly with increasing stirring speed but increases significantly with the increase of specific surface area, temperature and ammonium chloride concentration.
2) The shrinking core model with diffusion control fits the experimental results, but it could not physically represent the leaching kinetics.
3) The grain model, which can successfully describe the dissolution of zinc in ammonium chloride solutions, is proposed since no reaction products occurred on the particle surfaces according to the analysis of SEM and BEM. The reaction rate constant has a linear relationship with the square of the inverse of the initial particle radius.
4) The apparent activation energy of the leaching reaction and reaction order with respect to ammonium chloride are determined as 161.26 kJ/mol and 3.5, respectively.
References
[1] ESPIARI S, RASHCHI F, SADRNEZHAAD S K. Hydrometallurgical treatment of tailings with zinc content [J]. Hydrometallurgy, 2006, 82(1-2): 54-62.
[2] NAVIDI KASHANI A H, RASHCHI F. Separation of oxidized zinc minerals from tailings: Influence of flotation reagents [J]. Minerals Engineering, 2008, 21(12-14): 967-972.
[3] BODAS M G. Hydrometallurgical treatment of zinc silicate ore from Thailand [J]. Hydrometallurgy, 1996, 40(1-2): 37-49.
[4] TERRY B, MONHEMIUS A J. Acid dissolution of willemite ((Zn, Mn)2SiO4) and hemimorphite (Zn4Si2O7(OH)2H2O) [J]. Metallurgical Transactions B, 1983, 14(3): 335-346.
[5] SOUZA A D, PINA P S, LIMA E V O, DA SILVA C A,  V A. Kinetics of sulphuric acid leaching of a zinc silicate calcine [J]. Hydrometallurgy, 2007, 89(3-4): 337-345.
V A. Kinetics of sulphuric acid leaching of a zinc silicate calcine [J]. Hydrometallurgy, 2007, 89(3-4): 337-345.
[6] WENDT W J. Ammonia, ammonium carbonate leaching of low grade zinc ores [J]. Engineering and Mining Journal, 1953, 154(9): 84-90.
[7] HARVEY T G. The hydrometallurgical extraction of zinc by ammonium carbonate: A review of the Schnabel process [J]. Mineral Processing and Extractive Metallurgy Review, 2006, 27(4): 231-279.
[8] DING Zhi-ying, YIN Zhou-lan, HU Hui-ping, CHEN Qi-yuan. Dissolution kinetics of zinc silicate (hemimorphite) in ammoniacal solution [J]. Hydrometallurgy, 2010, 104(2): 201-206.
[9] ABDEL-AAL E A. Kinetics of sulfuric acid leaching of low-grade zinc silicate ore [J]. Hydrometallurgy, 2000, 55(3): 247-254.
[10] SANTOS F M F, PINA P S, PORCARO R, OLIVEIRA V A, SILVA C A,  V A. The kinetics of zinc silicate leaching in sodium hydroxide [J]. Hydrometallurgy, 2010, 102(1-4): 43-49.
V A. The kinetics of zinc silicate leaching in sodium hydroxide [J]. Hydrometallurgy, 2010, 102(1-4): 43-49.
[11] CHEN Ai-liang, ZHAO Zhong-wei, JIA Xi-jun, LONG Shuang, HUO Guang-sheng, CHEN Xing-yu. Alkaline leaching Zn and its concomitant metals from refractory hemimorphite zinc oxide ore [J]. Hydrometallurgy, 2009, 97(3-4): 228-232.
[12] LIMPO J L, FIGUEIREDO J M, AMER S, LUIS A. The CENIM- LNETI process: A new process for the hydrometallurgical treatment of complex sulphides in ammonium chloride solutions [J]. Hydrometallurgy, 1992, 28(2): 149-161.
[13] HE Jing, HUANG Ling, CHEN Yong-ming, TANG Mo-tang, JIN Sheng-ming, FENG Rui-zhu, WU Sheng-nan. Solvent extraction of zinc from Zn(II)-NH3 complex system by new extractant YORS [J]. The Chinese Journal of Nonferrous Metals, 2011, 21(3): 687-693. (in Chinese)
[14] XIA Zhi-mei, YANG Sheng-hai, TANG Mo-tang, YANG Tian-zu, LIU Zhi-hong, TANG Chao-bo, HE Jing, DENG Xiao-ling. Cycle leaching of low grade zinc oxide ores in MACA system for preparing zinc [J]. The Chinese Journal of Nonferrous Metals, 2013, 23(12): 3455-3461. (in Chinese)
[15] LI Yan, HUA Yi-xin, LIN Zuo-yan. A novel process for synthesis of zinc silicate [J]. Journal of Materials and Metallurgy, 2007, 6(3): 224-229. (in Chinese)
[16] GEORGIOU D, PAPANGELAKIS V G. Sulphuric acid pressure leaching of a limonitic laterite: Chemistry and kinetics [J]. Hydrometallurgy, 1998, 49(1-2): 23-46.
[17] JU Shao-hua, TANG Mo-tang, YANG Sheng-hai, LI Ying-nian. Dissolution kinetics of smithsonite ore in ammonium chloride solution [J]. Hydrometallurgy, 2005, 80(1-2): 67-74.
[18] FILIPPOU D, KONDURU R, DEMOPOULOS G P. A kinetic study on the acid pressure leaching of pyrrhotite [J]. Hydrometallurgy, 1997, 47(1): 1-18.
[19] RAGHAVAN S, GAJAM S Y. Application of an enlarging pore model for the ammoniacal leaching of chrysocolla [J]. Hydrometallurgy, 1986, 16(3): 271-281.
[20] LIMPO J L, LUIS A. Solubility of zinc chloride in ammoniacal ammonium chloride solutions [J]. Hydrometallurgy, 1993, 32(2): 247-260.
[21] SOUZA A D, PINA P S, SANTOS F M F, DA SILVA C A,  V A. Effect of iron in zinc silicate concentrate on leaching with sulphuric acid [J]. Hydrometallurgy, 2009, 95(3-4): 207-214.
V A. Effect of iron in zinc silicate concentrate on leaching with sulphuric acid [J]. Hydrometallurgy, 2009, 95(3-4): 207-214.
硅酸锌在氯化铵溶液中的浸出动力学
杨声海,李 浩,孙彦伟,陈永明,唐朝波,何 静
中南大学 冶金与环境学院,长沙 410083
摘 要:研究硅酸锌在氯化铵溶液中的浸出动力学,讨论搅拌速度(150~400 r/min)、浸出温度(95~108 °C)、硅酸锌粒度(61~150 μm)以及氯化铵浓度(3.5~5.5 mol/L)对锌浸出率的影响。结果表明,减小硅酸锌粒度、提高浸出温度和氯化铵浓度可以显著地提高锌的浸出率。在多孔颗粒的动力学模型中,颗粒模型的孔隙扩散控制能很好地描述锌的浸出动力学。浸出反应的表观活化能为161.26 kJ/mol,氯化铵的反应级数为3.5.
关键词:硅酸锌;氯化铵;浸出;动力学;孔隙扩散控制
(Edited by Wei-ping CHEN)
Foundation item: Project (2014CB643404) supported by the National Basic Research Program of China; Project (51374254) supported by the National Natural Science Foundation of China
Corresponding author: Sheng-hai YANG; Tel: +86-13975894838; E-mail: yangsh@mail.csu.edu.cn
DOI: 10.1016/S1003-6326(16)64278-4
Abstract: The leaching kinetics of zinc silicate in ammonium chloride solution was investigated. The effects of stirring speed (150-400 r/min), leaching temperature (95-108 °C), particle size of zinc silicate (61-150 μm) and the concentration of ammonium chloride (3.5-5.5 mol/L) on leaching rate of zinc were studied. The results show that decreasing the particle size of zinc silicate and increasing the leaching temperature and concentration of ammonium chloride can obviously enhance the leaching rate of zinc. Among the kinetic models of the porous solids tested, the grain model with porous diffusion control can well describe the zinc leaching kinetics. The apparent activation energy of the leaching reaction is 161.26 kJ/mol and the reaction order with respect to ammonium chloride is 3.5.


