Trans. Nonferrous Met. Soc. China 24(2014) 1220-1230
Penetrative and migratory behavior of alkali metal in different binder based TiB2-C composite cathodes
Zhao FANG, Xiao-lei WU, Juan YU, Lin-bo LI, Jun ZHU
School of Metallurgical Engineering, Xi’an University of Architecture and Technology, Xi’an 710055, China
Received 27 March 2013; accepted 27 May 2013
Abstract:
In electrolyte melts containing K at low temperature, the penetrative and migratory path of alkali metals (K and Na) in pitch, furan, phenolic aldehyde and epoxy based TiB2-C composite cathodes during the electrolysis process were studied by EDS and self-made modified Rapoport apparatus. The electrolysis expansion rates, the diffusion coefficients of the alkali metals and the corrosion rates of the composite cathode were also calculated and discussed. The results show that no matter what kind of binder is used, alkali metals have the same penetrative path in composite cathodes: firstly in pore, then in binder and finally in carbonaceous aggregates. K and Na penetrate into both binder and carbonaceous aggregates, which leads to the expansion of composite cathodes, and K has stronger penetration ability than Na. Electrolysis expansion rate of resin based composite cathode is smaller than that of pitch based composite cathodes, and so do the diffusion coefficient and corrosion rate. Resin based composite cathode has better resistance ability to the penetration of alkali metals than pith based composite cathode, and phenolic aldehyde based composite cathode exhibits the strongest resistance ability. The penetration rate, the diffusion coefficient of alkali metals in phenolic aldehyde based TiB2-C composite cathode and the corresponding corrosion rate are 4.72 mm/h, 2.24×10-5 cm2/s and 2.31 mm/a, respectively.
Key words:
aluminum electrolysis; penetration; migration; alkali metal; TiB2-C composite cathode; corrosion resistance;
1 Introduction
The new technique for aluminum production based on the inert electrode is expected to change conventional production process and achieve energy-saving by a large margin and the zero emissions of greenhouse gases during electrolysis process [1,2]. As an important component of the inert electrode system, the wettable cathodes have an important impact on the promotion of the industrial application of inert electrode system. However, as far as the current TiB2-C composite cathode is concerned, the problems of the short service life and easy to crack have not been solved thoroughly, which can not meet the requirements of the inert electrode system, and become obstacles to its industrial application [3-5].
TiB2 based wettable composite cathode is composed of TiB2 (function material), carbonaceous aggregates and binder, and roasting is one of its preparation procedures. After roasting, the composite cathode can be obtained. The carbonaceous aggregates in the composite cathode have good stability in the aluminum electrolysis process, since they have been heat-treated at higher temperature before the roasting of the green compact. Also, the TiB2. in the composite cathode has strong corrosion resistance, the character and structure of which don’t change in the roasting process. So, binder has become the most vulnerable weakness in the composite cathode [6,7]. At present, the most common binder used in the cathode is coal tar pitch. After carbonization, the microstructure of pitch changes to turbostratic graphite [8,9], the layer spacing of which is larger than that of the pure graphite. So, alkali metal is easy to penetrate into the interlayer irregularly. Moreover, under the bonding action between the electron in orbit s of alkali metal and the π electron in carbon, the corresponding reaction occurs and the thin layer embedded compounds ([CxM(K,Na)]) are generated [10], resulting in the enlargement of the interlayer spacing. And the expansion and failure phenomenon of cathode can be observed in macroscopic view. In addition, under the condition of industrial practice, liquid aluminum will react with the carbonaceous components in composite cathode to generate Al4C3, leading to the corrosion of cathode. In the interface of the cathode and the liquid aluminum, the generation and dissolution of the Al4C3 happen simultaneously, and finally reach balance. Al4C3 is difficult to dissolve in liquid aluminum, but it is easy to dissolve in the electrolyte. If the electrolyte exists in the interface, the dissolution of the Al4C3 will break the balance and induce the generation of new Al4C3. Once the new Al4C3 generates, it will be dissolved in the electrolyte again. As this phenomenon is inevitable, carbon binder phase in TiB2 based composite cathode will be eroded and consumed, inducing the aggregates in the composite cathode break off, and finally resulting in the failure of the cathode [11,12].
Corrosion resistance of the composite cathode has great effects on the service life of aluminum reduction cell, and the macroscopic properties of any materials depend on its microscopic structure and chemical bonding type. So, the most important problem about the TiB2-C composite cathode is to clarify the relationship between the macroscopic properties and the microscopic structure, and that is the key to make composite cathode have good wettability and excellent penetration resistance.
Around this problem, some related researches have been carried out at home and abroad. ADHOUM et al [13] studied the electrochemical intercalation of alkali metal in NaF melts at the temperature of 1025 °C and confirmed the two mechanisms of sodium intercalation: sodium precipitated on the cathode surface during the electrolysis process partly inserts into the interlayer of the graphite and partly penetrates into the pore of cathode materials both of them would generate graphite intercalation compound eventually. BRILLOIT et al [14] studied the penetration of alkali metal in carbon cathode. The results showed that sodium (adsorbed or inserted) was the main substance penetrated into the cathode, and its penetration rate and the saturation concentration in the cathode would increase with the increases of cryolite ratio (CR), while decline with the increase of carbon’s graphitization degree. The sodium penetrated into cathode could act as wetting agent and make the electrolyte penetrate into the cathode more easily, and then exacerbate the corrosion of cathode. LIU et al [15] studied the electrochemical intercalation of K into graphite cathode in KF melt, and the results revealed that K only intercalated into the spaces of graphite layers but had a certain degree of denudation effect on cathode. While FENG [16] found that sodium mainly diffused through crystal lattice when the porosity of carbon cathode was low, and it diffused through the pores when the porosity was high. However, these researches did not conduct online, but only tested the samples obtained after the electrolysis. At this circumstance, the existing form of alkali metal cannot completely represent the form of alkali metal in electrolysis process indeed. Moreover, the alkali metal penetration in cathode is a dynamic process. So, study of the process of alkali metal penetration into cathode and the migration path of electrolyte and alkali metal in the cathode would contribute to improve the corrosion resistance of cathode materials.
In this work, TiB2-C composite cathodes were employed as the research objects. Under the condition of different electrolysis time, the electrolytic expansion and corrosion rate of pitch, furan, phenolic aldehyde and epoxy based TiB2-C composite cathodes were investigated in low-temperature [K3AlF6/Na3AlF6]- AlF3-Al2O3 melts. The diffusion coefficients of alkali metal in the corresponding composite cathode were calculated and discussed. On the basis of above, the penetrative and migratory behavior of alkali metal (K and Na) in TiB2-C composite cathode was studied, and the penetrative and migratory paths of K and Na were analyzed.
2 Experimental
2.1 Materials and preparation of specimens
The composition of TiB2-C composite cathode is listed in Table 1, in which the size of TiB2 powder and petroleum coke are 12 μm and 106-270 μm, respectively. The binders used in this work were pitch, furan, phenolic aldehyde and epoxy. The involved chemical reagents such as K3AlF6, Na3AlF6, Al2O3 and AlF3 were all reagent grade. The values of CR (cryolite ratio), KR (potassium cryolite ratio) and tL (liquidus temperature) of the electrolyte melts are 1.6, 0.3 and 873 °C, respectively.
Table 1 Composition of TiB2-C composite cathodes (mass fraction, %)
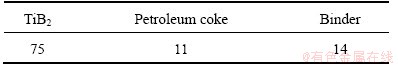
First, a certain proportion of TiB2 powder and petroleum coke were put into the three-dimensional motion kneader for mixing and kneading, and then mixed with binder. After weighing, the materials were molded by the universal hydraulic testing machine under the molding pressure of 150 MPa. The obtained cathodes were d20 mm×50 mm. Finally, cathode samples were put into the corundum crucible and covered by coke powder, then the crucible was placed into a program controlled chamber electric furnace and heated according to the temperature schedule, as shown in Fig. 1, to obtain the so-called TiB2-C composite cathodes.
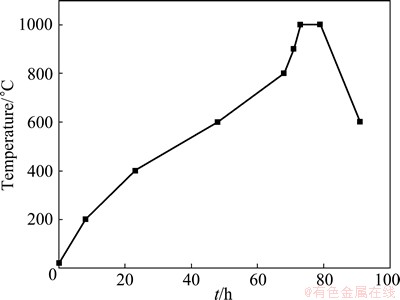
Fig. 1 Heating curves of roasting process for preparing TiB2-C composite cathodes
2.2 Experimental devices and methods
The research methods about the penetrative and migratory behavior of alkali metals in the cathode are mentioned as follows. The specimen was put into a cell made of high purity graphite in the vertical tube furnace, and the specimen and cell were separated by a corundum plate. The cell was filled with molten electrolyte, and the specimen was immersed into the molten electrolyte by 25 mm. Five electrolysis times (5, 15, 30, 60 and 120 min) were arranged for each experiments with different cathode to study the penetrative and migratory behavior of alkali metal in different cathodes. The concentration of alumina in the electrolyte was saturated in every experiment. The current density (ρCD) was 0.8 A/cm2 and the superheat temperature (tS) was 20 °C, The whole experimental process was taken in the high-purity argon atmosphere. A self-made modified Rapoport apparatus was used to test the electrolysis expansion rate of the specimen and the diffusion coefficient of alkali metals.
After electrolysis, the specimen was taken out and splitted along the radial direction. The cross section was about 10 mm above the bottom. Filter paper soaked with phenolphthalein was used to test the penetration rate of alkali metal in TiB2-C composite cathode. X-ray energy dispersive spectrometry (Noran Vantage4105) was used to test the element distribution in the cross section of specimens. X-ray fluorescence (Philips 8424 TW2424) was used to analyze the Ti content in the aluminum before and after electrolysis and then the corrosion rate of cathode was obtained accroding to the formula as follows:
 (1)
(1)
where Wloss is the corrosion rate of cathode (mm/y); wb is the mass of aluminium on the cathode after electrolysis (g); cb is the Ti content in the aluminium on the cathode after electrolysis (10-6); wa is the mass of aluminium on the cathode before electrolysis (g); ca is the Ti content in the aluminium on the cathode before electrolysis (10-6); h is the height of cathode specimen (mm); MTB is the molar mass of TiB2 (69.49 g/mol); MT is the molar mass of Ti (47.87 g/mol); wc is the total mass of the cathode; nT is the TiB2 content of the cathode; t is the electrolysis time.
3 Results and discussion
3.1 Penetration resistance of different binder based TiB2-C composite cathode
During the process of electrolysis, because of the polarization effects, alkali metals (K and Na) will precipitate on the cathode. Some of the obtained alkali metals dissolve in the liquid aluminum, some volatilize out in the form of steam and the rest will penetrate into the cathode, then react with the carbonaceous components, and form graphite-alkali metal intercalation compound [CxM(K,Na)]. Since these compounds are very unstable, so they can easily react with the moisture in the air to form sodium hydroxide. Therefore, phenolphthalein method can be used to determine the penetration front edge of the alkali metals (Fig. 2) and the corresponding penetration rate can be calculated [17].
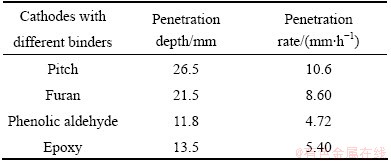
Table 2 shows the penetration depth and rate of alkali metals in pitch, furan, phenolic aldehyde and epoxy based TiB2-C composite cathodes according to the data in Fig. 2. It can be seen that, from low to high, the penetration rate of alkali metals is successive phenolic aldehyde, epoxy, furan and pitch. Penetration rate of alkali metal in resin based TiB2-C composite cathode is lower than that in pitch based TiB2-C composite cathode, suggesting that the penetration resistance of resin based TiB2-C composite cathode is superior to that of pitch based TiB2-C composite cathode. In addition, as for resin based TiB2-C composite cathode, the penetration depth and rate of alkali metal in phenolic aldehyde based TiB2-C composite cathode are the smallest, which decrease by 55.47%, compared with pitch based TiB2-C composite cathode.
As we know that pitch belongs to soft carbon materials, the pores formed during the pyrolysis process are mainly meso-pores or macro-pores, and the structure of the pitch phase is turbostratic graphite after carbonization. The turbostratic structure is vulnerable to the penetration and erosion of alkali metals (K and Na) during the aluminum electrolysis process. While, furan, phenolic aldehyde and epoxy belong to hard carbon materials, the pores formed during the pyrolysis process are mainly nano-pores. What’s more, with the increasing of temperature, the phenomenon of cross-linking will take place, leading to the formation of the rigid three-dimensional network structure and the alkali metals are different to penetrate. In macroscopic view, the penetration resistance of resin based TiB2-C composite cathode is better than that of pitch based TiB2-C composite cathode, and then the former can resist the destructive power of alkali metals better. According to the experimental results, the penetration resistance of phenolic aldehyde based TiB2-C composite cathode is the best.
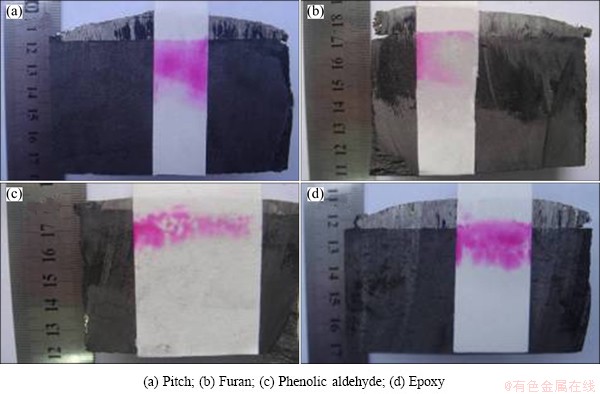
Fig. 2 Penetration front edge of alkali metals in different binder based TiB2-C composite cathode
Table 2 Penetration depth and rate of alkali metals in different kinds of cathode
3.2 Electrolysis expansion performance of TiB2-C composite cathode prepared by different binder
During electrolysis process, the continuous penetration of alkali metals into TiB2-C composite cathode will inevitably lead to the expansion of composite cathode. Figure 3 shows the electrolysis expansion curves obtained under the condition of different electrolysis time and different binder based TiB2-C composite cathodes in K-containing low temperature electrolyte melts. It can be seen from the figures that, no matter what kind of binder is used, the electrolysis expansion can be observed when the electrolysis continues for only 5 min. At this time, no K and Na are detected in the carbonaceous aggregates of the composite cathode, but in binder phase, some K and Na have been found. So, it can be concluded that, after electrolysis, the K and Na elements penetrated into the binder phase must be in the form of alkali metal. These alkali metals react with carbon, form corresponding intercalation compounds and finally result in the expansion of cathode. So, the electrolysis expasion of cathode is induced by the intercalation of K and Na into the binder coke at the initial stage of electrolysis.
With the extension of electrolysis time, K and Na gradually penetrate into the carbonaceous aggregates of composite cathode, and the expansion of cathode is caused mainly by the expansion of both binder coke and carbonaceous aggregates. In addition, Figure 3 also shows that, electrolytic expansions of pitch, furan, phenolic aldehyde and epoxy based TiB2-C composite cathodes after 2 h electrolysis are 1.35%, 1.09%, 0.85% and 0.92%, respectively, the electrolysis expansion of resin based TiB2-C composite cathodes is less than that of pitch based TiB2-C composite cathodes.
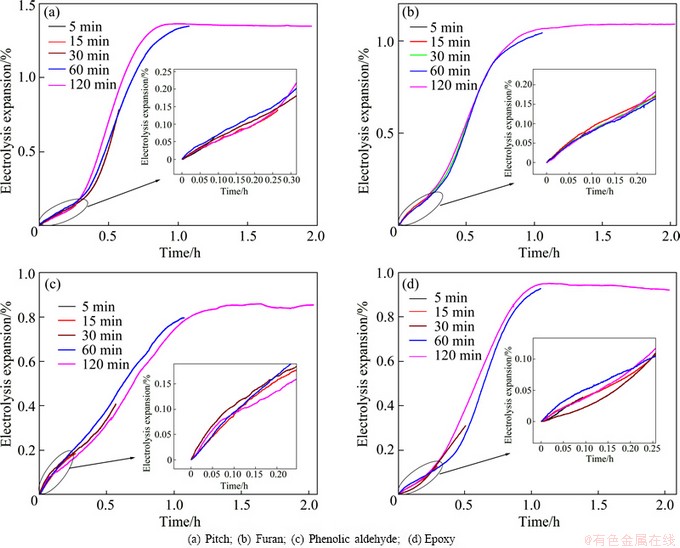
Fig. 3 Electrolysis expansion of different binder based TiB2-C composite cathodes
The penetrative and migratory process of alkali metals in TiB2-C composite cathode is a unsteady state diffusion process, so according to Fick’s second law, ZOLOCHEVSKY et al [18] proposed a constitutive model to describe the electrolysis expansion of cylinder cathode samples and calculate the diffusion coefficient (D) of alkali metals in the cathode during the electrolysis process. Alkali metals diffuse from the outer surface of cylinder cathode with radius b into the binder phase, so the concentration C of alkali metals in the cylinder can be expressed as
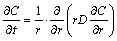 (2)
(2)
where r is the radial coordinate and t is the time. With the boundary and initial conditions:
C = C0, r = b, t ≥ 0
C = 0, 0>r>b, t = 0
The solution to Eq. (2) is
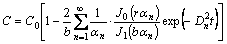 (3)
(3)
where J0(x) and J1(y) are the Bessel function of the first kind of zero order and the Bessel function of the first kind of the first order, respectively; and αn (n=1, 2, …) are the roots of J0(bαn)=0.
The solution to Eq. (3) can be written as
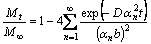 (4)
(4)
where Mt is the quantity of diffusing sodium penetrated into the cylinder at time t; M∞ is the sodium after infinite time. So, the diffusion coefficient (D) can be calculated by equation as follows:
 (5)
(5)
where t1/2 is the time to reach half of the saturation concentration in a cylinder with a radius r. Here, the concentration of alkali metals is proportional to the electrolysis expansion, so t1/2 can be obtained from Fig. 3.
Table 3 lists the alkali metals diffusion coefficients of different binders based TiB2-C composite cathodes.
Table 3 Diffusion coefficients and relevant parameters of alkali metals in different binder based TiB2-C composite cathodes

According to Table 3, the diffusion coefficient of alkali metals in pitch based TiB2-C composite cathode is maximal, which is 2.86×10-5 cm2/s. While in resin based TiB2-C composite cathode, the diffusion coefficient of alkali metals is smaller, especially in phenolic aldehyde based TiB2-C composite cathode, the diffusion coefficient of alkali metals is minimal, which is 2.24×10-5 cm2/s. After carbonization, resin mostly exhibits the three dimensional cross linking structure, while pitch shows turbostratic graphite structure [19]. This is the reason why the diffusion coefficient of alkali metals in pitch based TiB2-C composite cathode is higher than that in resin based TiB2-C composite cathodes. The diffusion coefficient of alkali metals is related to corrosion resistance of the cathode. When the diffusion coefficient of alkali metals is larger, the caused destructive power of the alkali metal is also larger, then the cathode will encounter serious corrosion, and vice versa.
3.3 Corrosion rate of different binder based TiB2-C composite cathodes
During aluminum electrolysis process, Reaction (6) occurs in the interface between liquid aluminum and the cathode, and the Al4C3 is formed [20]. In addition, the generated K and Na in the interface between molten electrolyte and liquid aluminum will diffuse through the liquid aluminum to the interface between liquid aluminium and the composite cathode, subsequently penetrate into the cathode. These alkali metals will react with the carbonaceous components, and form intercalation compound [CxM(K,Na)] [21] and Al4C3 according to Reaction (7). TiB2 has a good wettability with liquid aluminum, but under the condition of polarization, liquid aluminum on the surface of the cathode still presents a certain degree of volatility (Fig. 4) [22], causing that some electrolyte is in touch with the composite cathode. Under this circumstance, Reaction (8) occurs and the carbonaceous components are consumed. The binder coke is most likely to react with K and Na accroding to Reactions (6) and (7). When these reactions happen, a part of TiB2 grains will break off and enter into the liquid aluminum on the surface of cathode, resulting in the increace of Ti content and the corrosion of the cathode, which is also the theoretical basis of Eq. (1).
4Al(l)+3C(s)=Al4C3(s) (6)
4Na3AlF6(l)+12Na(l)+3C(s)=Al4C3(s)+24NaF(s) (7)
Al4C3(s)+5AlF3(l)+9NaF(l)=3Na3Al3CF8(l) (800 °C-1050 °C) (8)
Table 4 shows the corrosion rate and the related parameters of different binder based TiB2-C composite cathode. It can be seen from the table that, the corrosion rate of pitch based TiB2-C composite cathode is the highest, which is 7.29 mm/a. While the corrosion resistance of resin based TiB2-C composite cathodes is superior to that of pitch based TiB2-C composite cathode. The corrosion rate of phenolic aldehyde based TiB2-C composite cathode is minimal, which is only the 31.69% of the corrosion rate of pitch based TiB2-C composite cathode.

Fig. 4 Schematic diagram about melts flow of electrolysis process
Table 4 Corrosion rate and related parameters of different binder based TiB2-C composite cathodes
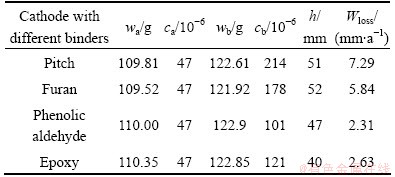
As for different binder based TiB2-C composite cathodes, the contact probability of the carbonaceous components with the liquid aluminum is equivalent during the electrolysis process, and so is the occurrence of Reactions (6) and (7). Also, the carbonaceous aggregates in each kind of composite cathode are identical. So, the difference of corrosion rate of different composite cathodes is induced only by the difference of the penetration resistance to alkali metals of the binder coke. The poorer the penetration resistance to alkali metal of the binder coke is, the more the penetration capacity of alkali metals into the cathode is. On the contrary, when the penetration resistance of binder coke is strong, the penetration capacity of alkali metals into the cathode is small; the quantity of Al4C3 generated by the Ractions (6) and (7) is also small. In macroscopic view, the corrosion rate of the cathode is low. It can be known from the discussion mentioned in Section 3.2 that, the penetration resistance of pitch based TiB2-C composite is the worst, while, that of phenolic aldehyde based TiB2-C composite cathode is the best. So, the corrosion rate of pitch based TiB2-C composite cathode is the highest, while, that of phenolic aldehyde based TiB2-C composite cathode is the lowest.
3.4 Penetrative and migratory path of alkali metals (K and Na) in different binder based TiB2-C composite cathodes
In general, the microstructure of TiB2-C composite cathode is shown as Fig. 5. It can be seen that composite cathode is composed of carbonaceous aggregates, pores, binder cokes and TiB2 particles. During the electrolysis process, as the penetration of alkali metals into the cathode is a diffusion process from outside to inside, EDS can be used to analyze the distribution of elements in the cross section of the composite cathode if the electrolysis time is controlled, and then the penetrative and migratory path of alkali metals into the cathode can be obtained.
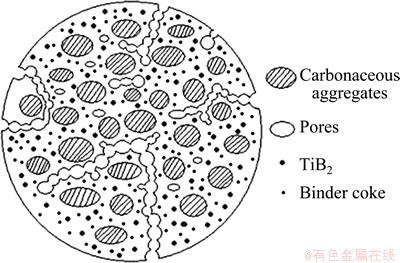
Fig. 5 Schematic diagram for microstructure of TiB2-C composite cathode
Figures 6-9 show the results about the element surface scanning of different binder based TiB2-C composite cathode after the electrolysis in K-containing low temperature electrolyte melts under different electrolysis time. The black region in BSE figures is carbonaceous aggregate particles, and the remaining region in the figures is the mixed region of TiB2 and binder phase. The particle sizes of the carbonaceous aggregates are controlled in the range of 106-270 μm, so that the penetration of alkali metals into different constituent of the cathode can be investigated conveniently after electrolysis.
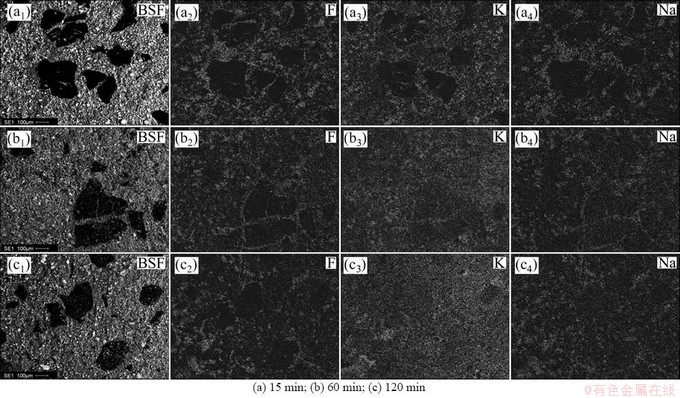
Fig. 6 Element mappings for cross section of pitch based TiB2-C composite cathode under different electrolysis time
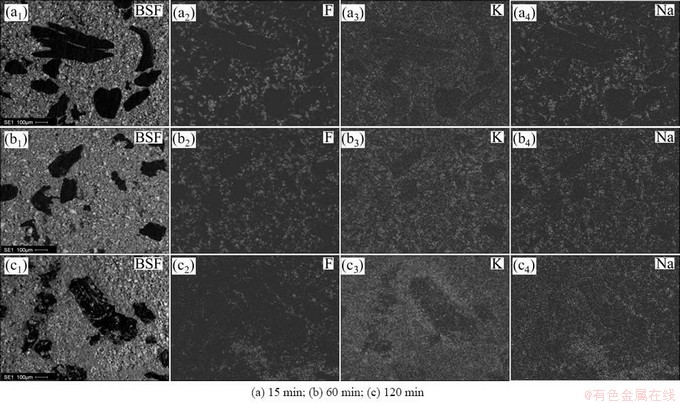
Fig. 7 Element mappings for cross section of furan based TiB2-C composite cathode under different electrolysis time

Fig. 8 Element mappings for cross section of phenolic aldehyde based TiB2-C composite cathode under different electrolysis time
As can be seen from Fig. 6 that, when the electrolysis continues for 15 min, the elements F, K and Na permeate into the mixed region of TiB2 and binder coke, that is, infiltrate into the binder phase of the cathode. No any other element can be detected in the TiB2, as shown in Fig. 10. But in the carbonaceous aggregates of the composite cathode, only a small amount of K and Na penetrate in and element F is not found. With the extension of electrolysis time, the content of the penetrated K and Na in the carbonaceous aggregates increases gradually, but, the penetration of element F is still not detected. This shows that the K and Na elements penetrated into the carbonaceous aggregates must be in the form of simple substance, and are likely to react with carbon to form corresponding intercalation compound [CxM(K,Na)]. If these part of K and Na is in the form of compound, i.e. the electrolyte, the element F should appear in the same region. When the electrolysis extends to 60 min, the elements F, K and Na permeate into binder phase continually. But in the carbonaceous aggregates, besides the penetration of a small amount of K and Na, no F element can be detected still. With the increase of electrolysis time, even to 120 min, K and Na are still the only elements penetrated into the carbonaceous aggregates, and element F is still not found. As can be seen from Figs. 7-9, the penetrations of F, K and Na into the cathode exhibit the similarity to Fig. 6.
According to the results in Figs. 6-9, the penetrative and migratory paths of alkali metals in different binder based TiB2-C composite cathodes during the electrolysis process can be obtained. With the proceeding of the electrolysis, alkali metals (K and Na) penetrate into the pores of the cathodes together with the electrolyte firstly, and then penetrate into the binder coke, finally penetrate into the carbonaceous aggregates of the cathode gradually. The penetration ability of K is stronger than that of Na. Alkali metals (K and Na) can not penetrate into the TiB2 particles. Alkali metals (K and Na) penetrated into both the binder coke and carbonaceous aggregates will lead to the electrolysis expansion of composite cathode. With the continuous penetration of alkali metals (K and Na), the electrolysis expansion of the cathode increases gradually, and finally becomes constant when the content of alkali metals (K and Na) in the cathode is saturated.
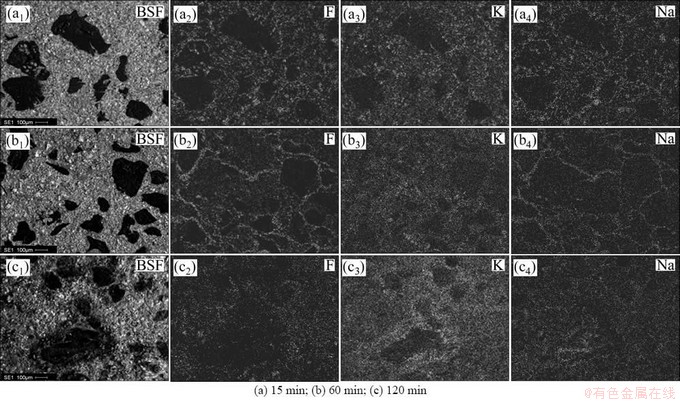
Fig. 9 Element mappings for cross section of epoxy based TiB2-C composite cathode under different electrolysis time
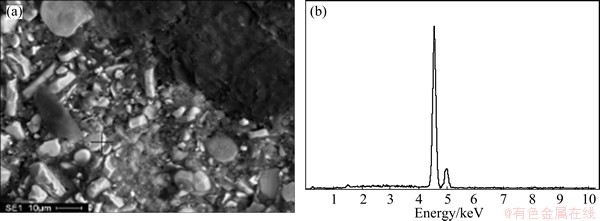
Fig. 10 SEM image (a) and EDS analysis (b) for TiB2 in composite cathode after electrolysis
In addition, from Figs. 6(c3) and (c4), it also can be seen that, after electrolysis of 120 min, because of the impact of the react order of intercalation compounds correspond to K and Na, the penetration capacity of K is obviously larger than that of Na. Meanwhile, the distribution of K or Na in any region of the cross section is almost the same, showing that the penetration resistance to alkali metals of pitch based TiB2-C composite cathode is poor, and which is more obvious compared with the results shown in Figs. 7(c), 8(c) and 9(c). It can be seen from Figs. 7(c) that, in different regions of the composite cathode, the content of the penetrated K or Na is different, the penetration capacity of K and Na in the binder phase is higher than that in the carbonaceous aggregates. Compared with Fig. 6(c), the penetration resistance to alkali metals of resin binder is stronger than that of pitch binder.
4 Conclusions
1) Owing to the difference of cathode’s microstructure, the penetration rates and the diffusion coefficients of alkali metals in various binders based TiB2-C composite cathodes are different. The penetration depth and rate of alkali metals in phenolic aldehyde based TiB2-C composite cathode are the smallest, which decrease by 55.47% compared with the pitch based TiB2-C composite cathode. The diffusion coefficient of alkali metals in resin based TiB2-C composite cathode is also smaller, especially in phenolic aldehyde based TiB2-C composite cathode, the diffusion coefficient is minimal, which is only 2.24×10-5cm2/s. The corrosion resistance of resin based TiB2-C composite cathode is superior to that of pitch based TiB2-C composite cathode, among which the corrosion rate of phenolic aldehyde based TiB2-C composite cathode is minimal, which is 2.31 mm/a, only 31.69% of the corrosion rate of the pitch based TiB2-C composite cathode.
2) The penetrative and migratory path of alkali metals in different binder based TiB2-C composite cathodes during the electrolysis process is as follows: with the proceding of the electrolysis, alkali metals (K and Na) firstly penetrate into the pores of the cathode together with the electrolyte, subsequently penetrate into the binder coke, finally penetrate into the carbonaceous aggregates of the composite cathode gradually. The penetration ability of K is stronger than that of Na. Alkali metals (K and Na) can not penetrate into the TiB2 particles. Alkali metals (K and Na) penetrated into both the binder coke and carbonaceous aggregates will lead to the electrolysis expansion of composite cathode. With the continuous penetration of alkali metals (K and Na), the electrolysis expansion of the cathode increases gradually, and finally becomes constant when the content of alkali metals (K and Na) in the cathode is saturated.
References
[1] Aluminum Association. Aluminum industry technology roadmap [M]. Aluminum Association, 2003.
[2] JAMES W E, HALVOR K. Sustainability, climate change, and greenhouse gas emissions reduction: Responsibility, key challenges, and opportunities for the aluminum industry [J]. JOM, 2008, 60(8): 25-31.
[3]  Xiao-jun, XU Jian, LI Jie, LAI Yan-qing, LIU Ye-xiang. Thermal-treated pitches as binders for TiB2/C composite cathodes [J]. Metallurgical and Materials Transactions A, 2012, 43(1): 219-227.
Xiao-jun, XU Jian, LI Jie, LAI Yan-qing, LIU Ye-xiang. Thermal-treated pitches as binders for TiB2/C composite cathodes [J]. Metallurgical and Materials Transactions A, 2012, 43(1): 219-227.
[4] HOU Jin-long,  Xiao-jun, ZHANG Hong-liang, LAI Yan-qing, LI Jie. Furan resin and pitch blends as binders for TiB2-C cathodes [C]//LINDSAY S J. Light Metals 2011. USA: TMS, 2011: 1117-1121.
Xiao-jun, ZHANG Hong-liang, LAI Yan-qing, LI Jie. Furan resin and pitch blends as binders for TiB2-C cathodes [C]//LINDSAY S J. Light Metals 2011. USA: TMS, 2011: 1117-1121.
[5] HEIDARI H, ALAMDARI H,  D, SCHULZ R. Pressureless sintering of TiB2-based composites using Ti and Fe additives for development of wettable cathodes [C]//LINDSAY S J. Light Metals 2011. USA: TMS, 2011: 1111-1116.
D, SCHULZ R. Pressureless sintering of TiB2-based composites using Ti and Fe additives for development of wettable cathodes [C]//LINDSAY S J. Light Metals 2011. USA: TMS, 2011: 1111-1116.
[6] IBRAHIEM M O, FOOSNAES T, OYE H A. Chemical stability of pitch-based TiB2-C coatings on carbon cathodes [C]//SORLIE M. Light Metals 2007. USA: TMS, 2007: 1041-1046.
[7] WANG Zhao-wen, BAN Yun-gang, SHI Zhong-ning, GAO Bing-liang,  Ding-xiong, MA Cheng-gui, KAN Hong-min, HU Xian-wei. Penetration of sodium and electrolyte to vibratory compaction TiB2 cathode [C]//DAVID H D Y. Light Metals 2008. USA: TMS, 2008: 1029-1032.
Ding-xiong, MA Cheng-gui, KAN Hong-min, HU Xian-wei. Penetration of sodium and electrolyte to vibratory compaction TiB2 cathode [C]//DAVID H D Y. Light Metals 2008. USA: TMS, 2008: 1029-1032.
[8] BARON J T, MCKINNEY S A, WOMBLES R H. Coal tar pitch-past, present, and future [C]//BEARNE G. Light Metals 2009. USA: TMS, 2009: 935-939.
[9] LI Jie, FANG Zhao,  Xiao-jun, TIAN Zhong-liang, LAI Yan-qing, XU Jian. Effects of superheat and current density on electrolysis expansion performance of semi-graphitic cathode at low temperature [J]. The Chinese Journal of Nonferrous Metals, 2009, 19(12): 2222-2229. (in Chinese)
Xiao-jun, TIAN Zhong-liang, LAI Yan-qing, XU Jian. Effects of superheat and current density on electrolysis expansion performance of semi-graphitic cathode at low temperature [J]. The Chinese Journal of Nonferrous Metals, 2009, 19(12): 2222-2229. (in Chinese)
[10] BRILLOIT P, LOSSIUS L P, OYE H A. Melt penetration and chemical reaction in carbon cathodes during aluminium electrolysis. I. Laboratory experiments [C]//SUBODH K D. Light Metals 1993. USA: TMS, 1993: 321-330.
[11] RAFIEI P, HILTMANN F, HYLAND M, JAMES B, WELCH B. Electrolytic degradation within cathode materials [C]//ANJIER J L. Light Metals 2001. USA: TMS, 2001: 747-752.
[12] STEVENSA D A, DAHNB J R. The mechanisms of lithium and sodium insertion in carbon materials [J]. Journal of Electrochemical Society, 2001, 148(8): 803-811.
[13] ADHOUM N, BOUTEILLON J, DUMAS D, POIGNET J C. Electrochemical insertion of sodium into graphite in molten sodium fluoride at 1025 °C [J]. Electrochimica Acta, 2006, 51(25): 5402-5406.
[14] BRILLOIT P, LOSSIUS L P, OYE H A. Penetration and chemical reactions in carbon cathodes during aluminium electrolysis. I. laboratory experiments [J]. Metallurgical Transactions B, 1993, 24(1): 75-89.
[15] LIU Dong-ren, YANG Zhan-hong, LI Wang-xing, QIU Shi-lin, LUO Ying-tao. Electrochemical intercalation of potassium into graphite in KF melt [J]. Electrochimica Acta, 2010, 55(3): 1013-1018.
[16] FENG Nai-xiang. Penetration of cryolite melts and sodium into cathode carbon blocks during electrolysis [J]. Acta Metallurgica Sinica, 1999, 35(6): 611-617. (in Chinese)
[17] OYE H A, NORA V D, DURUZ J J. Properties of a colloidal alumina-bonded TiB2 coating on cathode carbon materials [C]// HUGLEN R. Light Metals 1997. USA: TMS, 1997: 279-286.
[18] ZOLOCHEYSKY A, HOP J G, SERVANT T, FOOSNAS T, OYE H A. Rapoport-samoilenko test for cathode carbon materials. II. Swelling with external pressure and effect of creep [J]. Carbon, 2005, 43(6): 1222-1230.
[19] JIANG Wen-zhong. Carbon technology [M]. Beijing: Metallurgical Industry Press, 2009: 141-142. (in Chinese)
[20] VASSHAUG K, FOOSNAES T, HAARBERG G M, RATVIK A P, SKYBAKMOEN E. Formation and dissolution of aluminium carbide in cathode blocks [C]//BEARNE G. Light Metals 2009, USA: TMS, 2009: 1111-1116.
[21] CLAIRE H, ALBERT H, PHILIPPE L. Ternary graphite intercalation compounds associating an alkali metal and an electronegative element or radical [J]. Solid State Sciences, 2004, 6(1): 125-138.
[22] SIEW E F, HAY T I, STEPHENS G T, CHEN J J. TAYLOR M P. A study of the fundamentals of pothole formation [C]//KVANDE H. Light Metals 2005. USA, TMS, 2005: 763-769.
不同粘结剂基TiB2-C复合阴极中碱金属的渗透迁移行为
方 钊,伍小雷,俞 娟,李林波,朱 军
西安建筑科技大学 冶金工程学院,西安 710055
摘 要:在含K低温电解质熔体中,采用EDS及改进型电解膨胀率测试仪,分别研究电解过程中沥青、呋喃、酚醛、环氧基TiB2-C复合阴极中碱金属(K和Na)的渗透迁移路径。同时,计算并讨论相应的电解膨胀率、碱金属的扩散系数以及复合阴极的腐蚀率。结果表明:无论使用何种粘结剂,电解过程中,碱金属在阴极中表现出相似的渗透迁移路径:碱金属首先渗透进入阴极的孔隙当中,随后渗透进入粘结剂相中,随着电解的不断进行,最后渗透进入复合阴极的骨料相当中。渗透进入粘结剂相和炭质骨料相当中的K和Na均会引起复合阴极的电解膨胀,同时,K比Na有着更强的渗透力。树脂基复合阴极的电解膨胀率、碱金属在其中的扩散系数以及腐蚀率均小于沥青基复合阴极,即,树脂基复合阴极的抗碱金属渗透力强于沥青基复合阴极。而就树脂基TiB2-C复合阴极而言,酚醛基TiB2-C复合阴极的抗渗透力最强,碱金属在其中的渗透速率、扩散系数和相应的腐蚀率分别为4.72 mm/h, 2.24×10-5 cm2/s和2.31 mm/a.
关键词:铝电解;渗透;迁移;碱金属;TiB2-C复合阴极;耐腐蚀
(Edited by Chao WANG)
Foundation item: Project (51304152) supported by the National Natural Science Foundation of China; Project (2013JQ7016) supported by the Natural Science Foundation of Shanxi Province, China; Project (2013JK0904) supported by Shanxi Provincial Education Department, China
Corresponding author: Zhao FANG; Tel: +86-29-82202923; E-mail: fangzhao889@126.com
DOI: 10.1016/S1003-6326(14)63182-4
Abstract: In electrolyte melts containing K at low temperature, the penetrative and migratory path of alkali metals (K and Na) in pitch, furan, phenolic aldehyde and epoxy based TiB2-C composite cathodes during the electrolysis process were studied by EDS and self-made modified Rapoport apparatus. The electrolysis expansion rates, the diffusion coefficients of the alkali metals and the corrosion rates of the composite cathode were also calculated and discussed. The results show that no matter what kind of binder is used, alkali metals have the same penetrative path in composite cathodes: firstly in pore, then in binder and finally in carbonaceous aggregates. K and Na penetrate into both binder and carbonaceous aggregates, which leads to the expansion of composite cathodes, and K has stronger penetration ability than Na. Electrolysis expansion rate of resin based composite cathode is smaller than that of pitch based composite cathodes, and so do the diffusion coefficient and corrosion rate. Resin based composite cathode has better resistance ability to the penetration of alkali metals than pith based composite cathode, and phenolic aldehyde based composite cathode exhibits the strongest resistance ability. The penetration rate, the diffusion coefficient of alkali metals in phenolic aldehyde based TiB2-C composite cathode and the corresponding corrosion rate are 4.72 mm/h, 2.24×10-5 cm2/s and 2.31 mm/a, respectively.


