Trans. Nonferrous Met. Soc. China 25(2015) 661-668
Corrosion wear synergistic behavior of Hastelloy C276 alloy in artificial seawater
Jun CHEN1,2,3, Jian-zhang WANG2, Feng-yuan YAN2, Qing ZHANG1,3, Quan-an LI1,3
1. School of Materials Science and Engineering, Henan University of Science and Technology, Luoyang 471023, China;
2. State Key Laboratory of Solid Lubrication, Lanzhou Institute of Chemical Physics, Chinese Academy of Sciences, Lanzhou 730000, China;
3. Collaborative Innovation Center of Nonferrous Metals of Henan Province, Luoyang 471023, China
Received 29 April 2014; accepted 10 September 2014
Abstract:
A systematic investigation was carried out to discuss the corrosion and tribocorrosion behaviors of Hastelloy C276 alloy sliding against Al2O3 pin in artificial seawater, using a pin-on-disk tribometer integrated with a potentiostat for electrochemical control. The results show that the great decrease of open circuit potential and three orders of magnitude increase of corrosion current density occur caused by friction. There are clearly synergistic effect between corrosion and wear, resulting in corrosion-induced-wear and wear-induced-corrosion in tribocorrosion process. The contribution of pure mechanical wear to total material loss exceeds 70% in all sliding conditions, so mechanical wear is the dominant factor during tribocorrosion. For considering synergistic effect between corrosion and wear, the contribution of wear-induced-corrosion to total material loss is not very high although corrosion rate is greatly accelerated by friction. The fraction of corrosion-induced-wear to the total material loss is high and in the range of 14.6%-20.5% under all sliding conditions.
Key words:
tribocorrosion; Hastelloy C276 alloy; synergistic effect; seawater;
1 Introduction
In many situations, passive alloys are subjected to combined corrosion and wear, which can be defined as tribocorrosion. These can lead to the destroy or even complete removal of passive film from contact surface, resulting in accelerated metal dissolution, which in turn causes accelerated wear loss [1-4]. Over past few years, investigation on tribocorrosion behaviors of materials subjected to corrosion and wear is a hot topic. Stainless steel and CoCrMo alloy are the most frequently used metals in tribocorrosion field [2,5].
Nickel-based alloys are widely used in chemical industry (vessels, pipes, heat-exchangers and pumps), automotive industries and marine industry (pumps, impellers, valves, and other kinds of equipments). Recently, the tribocorrosion behavior of Ni-Cr alloy was investigated by researchers. The tribocorrosion behavior of overlay welded Ni–Cr 625 alloy in sulfuric and nitric acids under potentiostatic was investigated by ESPALLARGAS and MISCHLER [6]. The tribo- corrosion behavior in sulfuric acid was similar to that of stainless steels found in other studies, with much lower wear at cathodic potential compared with passive conditions. However, wear behavior in nitric acid was highly influenced by the reduction reaction of nitric acid at the electrode–electrolyte interface, which leads to the oxidation of the alloy even at cathodic potential. BI et al [7] studied the tribocorrosion property of Ni-17.5Si- 29.3Cr alloy sliding against a Si3N4 ball in comparison with AISI 321 stainless steel in 1 mol/L H2SO4 solution. The results indicated that the alloy showed excellent corrosion resistance and anti-wear ability compared with AISI 321 stainless steel. However, the fundamental mechanism that determines the wear-corrosion synergism in tribocorrosion of Ni-based alloy in chloride-containing solution has not been fully understood.
The aim of this work is to assess the tribocorrosion behavior of Ni-Cr alloy (Hastelloy C276) in artificial seawater. The counterpart material is alumina, which is considered to act as an inert antagonist in this experiment. Ceramic materials have excellent insulation, high hardness and good wear resistance, making them suitable for investigating the tribocorrosion properties of metals as counterpart materials. Corrosion wear experiments were carried out in a developed apparatus allowing for well-controlled mechanical and electrochemical conditions.
2 Experimental
2.1 Materials and solutions
The researched metallic alloy in this experiment was Hastelloy C276 nickel-based alloy with the following chemical compositions: 16% Cr, 16% Mo, 5% Fe, 4% W, 2.5% Co, 1% Mn, 0.35% V, 0.08% Si, 0.01% C, balance Ni. The alloy was machined into a ring with an outer diameter of 54 mm and an inner diameter of 38 mm. And only upper surface of the alloy was in touch with seawater and other surfaces were covered with paint for insulation to avoid electrochemical test confusion. The Al2O3 pin with a flat surface at one end of a cylinder (diameter: 4.7 mm, height: 13 mm) was used as counterpart material. All samples were polished down to mirror quality using 220, 500 and 1000 grade grit paper and with 1 μm diamond suspension, respectively. Corrosive solution was artificial seawater prepared according to ASTM D1141-98 standard. The pH value was 8.2. All tests were carried out at room temperature (20-25 °C).
2.2 Wear measurements
Corrosion wear test setup combined with in-situ electrochemical measurements was illustrated in our previous paper. Sliding tests were carried out using an MMW-1 pin-on-disk tribometer. In-suit electrochemical measurements during sliding were fulfilled using CHI760C potentiostat. Wear track was a ring with a mean diameter of 46 mm and a width of 4.7 mm. The area of wear track was about 6.8 cm2. The rotation speed in this experiment was 100-400 r/min with linear velocity of 0.27-1.08 m/s and normal load was 100-200 N. After corrosion-wear tests, the metallic specimens were ultrasonically cleaned in acetone to remove debris. Gravimetric measurement before and after tribocorrosion were finished. The volume loss can be calculated as
 (1)
(1)
where V is the volume loss (mm3), m0 is the mass of material before wear (mg), m1 is the mass of material after wear (mg), and ρ is density of the alloy. The specific wear rate, k, can be determined as
V=kLN (2)
where N is the applied load and L is the total sliding distance. The morphologies of worn surfaces were examined using JEM-5600LV scanning electron microscope (SEM). All tests were repeated at least three times for reproducibility.
2.3 Electrochemical measurements
Electrochemical measurements were concluded using a CHI760C potentiostat with three-electrode test system. Metallic specimen was prepared as working electrode (WE). A saturated calomel electrode (SCE) close to working electrode served as reference electrode (RE) and platinum was used as counter electrode (CE). All potentials in this work are given with respect to the saturated calomel electrode (SCE). Several series of experiments were conducted to study corrosion-wear synergistic behaviors of the alloy: 1) Potentiodynamic test involving measuring polarization curves was initiated after a stable open potential. It was fulfilled with a potential sweep starting at -1 V and finishing at 1 V at a sweep rate of 1.67 mV/s. CHI software was used to analyze polarization data; 2) Corrosion-wear tests were carried out at open circuit potential and the evolution of open circuit potential was measured; 3) To analyze the synergistic effect between corrosion and wear, sliding wear tests polarized at -0.8 V were conducted and the transient current was measured.
3 Results and discussion
3.1 Electrochemical behavior
The evolution of open circuit potential (OCP) of Hastelloy C276 alloy under tribocorrosion is shown in Fig. 1. It can be obtained that at the start of sliding, open potential drops sharply down to more negative value due to friction. The cathodic shift phenomenon of OCP during corrosion-wear is frequently observed for stainless steel, TC4 and Co-Cr alloys by many researchers [8]. It can be expounded by the removal or damage of passive oxide film formed on original surface because of friction [9,10]. When friction stops, OCP starts to increase (anodic shift) abruptly and then more gradually reaches initial OCP without sliding about -0.2 V. This indicates the re-establishment of passive state in wear track.
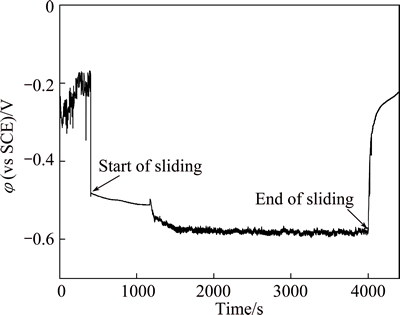
Fig. 1 Evolution of OCP of Hastelloy C276 alloy under tribocorrosion
Generally, OCP measurement enables to gather electrochemical information in wear track and can easily evaluate the electrochemical behavior of metal surface. However, this electrochemical technique is powerless for assessing corrosion kinetics. Potentiodynamic polarization method was used to evaluate corrosion kinetics during tribocorrosion in this experiment [9].
The polarization curves of Hastelloy C276 alloy under corrosion-only and sliding conditions are shown in Fig. 2. Passive phenomenon and very low passive current density can be seen under corrosion-only, which is comparable with commonly stainless steel and titanium alloys, suggesting strong passivation ability [8,11]. The polarization curves obtained during tribocorrosion reflect corrosion behavior and repassivating ability when passive film is mechanically damaged or removed by friction. Since contact area of friction couples is constant in this experiment, the calculation of current density is based on the area of wear track. The polarization curve exhibits significant current oscillations, which can be attributed to instability of corrosion-wear system and simultaneous interaction of damage and recovery of passive film. Moreover, contact positions of friction couples change continuously, which also contributes to oscillations of curves [12,13]. The corrosion potential in tribocorrosion shifts to lower value, more active potentials by about 0.3 V compared with that under corrosion-only condition, which is consistent with result of OCP measurement. Apparent passive region is also observed, which means that Hastelloy C276 alloy exhibits passivation performance even in tribocorrosion. Importantly, corrosion current density during sliding is increased by three orders of magnitude compared with that under corrosion-only condition. It can be concluded that corrosion rate is significantly increased by friction in this experiment. The degradation of passive film causes high dissolution of metal, resulting in wear-induced- corrosion.
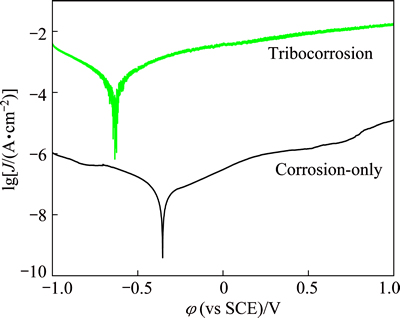
Fig. 2 Polarization curves of Hastelloy C276 alloy under corrosion-only and sliding conditions
The corrosion current I measured in polarization curves during tribocorrosion can be considered the sum of two parts of currents Ia and Ip:
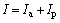 (3)
(3)
where Ia is the current produced from wear track, and Ip is current produced from unworn area. For the unworn area, Ip cannot exceed current value measured at the same potential in corrosion-only curves. By comparing the values of Ip and Ia around corrosion potential, it can be concluded that I is almost equal to Ia. The measured corrosion current and its evolution with potential during tribocorrosion, are characteristic of the electrochemical behavior in wear track [9].
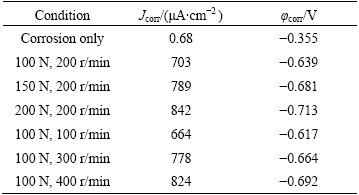
Table 1 shows corrosion potential (φcorr) and the corresponding corrosion current density (Jcorr). The Jcorr was determined using Tafel extrapolation method. The current density increases obviously with the increase of normal load and rotation speed, which is much larger than that under corrosion-only condition. High normal load and rotation speed result in low open circuit potential and high corrosion current density. Rotation speed is linked to the time interval of two successive contact events during sliding. Increasing contact frequency causes less time available for repassivation between contact intervals. Therefore, the amount of active area in wear track is large at high rotation speed, which results in low corrosion potential and high corrosion current density. The increase of corrosion current density with increasing normal load can be explained by the severely damaged passive film, severe plastic deformation and high densities of defects [9,14].
Table 1 Corrosion potential (φcorr) and current density (Jcorr) for Hastelloy C276 alloy
3.2 Wear behavior
Corrosion rate is significantly enhanced by friction and synergistic effect between wear and corrosion results in accelerated material loss. In order to determine the contribution of the synergy to total material loss, wear test was conducted at a cathodic potential of -0.8 V, which is lower than the corrosion potentials measured under both tribocorrosion and corrosion-only conditions. Under this electrochemical condition, there is no electrochemical corrosion and material loss is only caused by mechanical wear. Similar test procedure was employed by other investigators to evaluate the mechanical wear component for many alloys [15,16]. Figure 3 shows the evolution of current density at -0.8 V under 100 N and 200 r/min. Clearly, the measured current is cathodic before, during and after sliding, confirming that no corrosion occurs.
Table 2 shows the wear loss, mean friction coefficient and specific wear rate (k) of Hastelloy C276 alloy under both OCP and cathodic potential conditions. It can be seen that wear loss is much greater at OCP than that at cathodic potential of -0.8 V. At OCP, both corrosion and wear take place, resulting in accelerated wear loss. Clearly, corrosion has a significant effect on wear of Hastelloy C276 alloy. It is obvious that wear loss increases with increasing normal load and rotation speed. The specific wear rate is an effective parameter when sliding condition is different. Specific wear rate is in the range of 10-4 mm3/(N·m) for Hastelloy C276 alloy. The wear rate decreases clearly with the increase of rotation speed. The average friction coefficient of Hastelloy C276 alloy is in the range of 0.45-0.52 and increases slightly with increase of normal load.
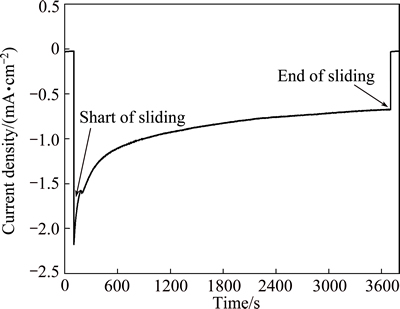
Fig. 3 Evolution of current density for Hastelloy C276 alloy at -0.8 V
Metal wear debris is transferred on the pin surface as shown in Fig. 4(a). The wear loss of Al2O3 pin is large and the surface is much roughened, as shown in Fig. 4(b). The roughened Al2O3 surface would abrade the metallic surface and lead to the transfer of opposing metallic debris, resulting in the severe wear. IWABUCHI et al [8,17] reported the same serious damage of Al2O3, which may be caused by cold welding. Such a rough surface of Al2O3 pin was also observed for the combination with Ni-alloy in this work.
3.3 Synergistic effect
In addition to the commonly observed phenomena related to the cathodic shift of OCP, the increase of corrosion current and the accelerated wear loss, several interesting phenomena deserve further discussion in the present experiment. These include correlation between materials loss and wear mechanism as well as the synergistic effect between corrosion and wear.
Table 2 Wear loss, mean friction coefficient and specific wear rate (k) of Hastelloy C276 alloy in seawater
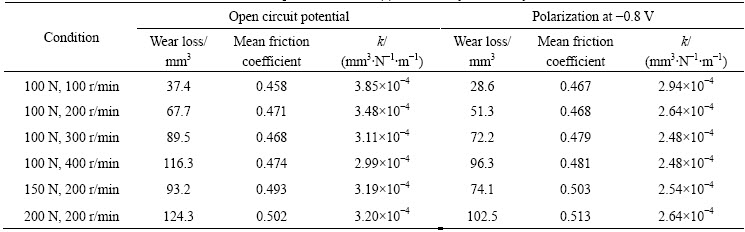
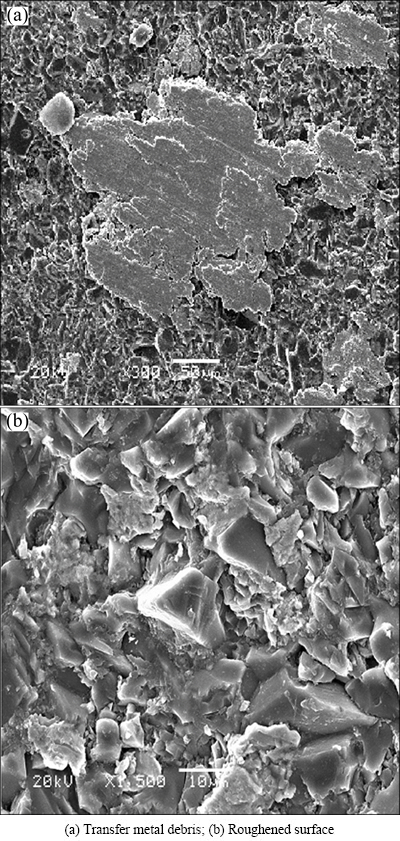
Fig. 4 SEM images of wear track on Al2O3 pin after friction test
Corrosion current density in Table 1 shows the existence of accelerated corrosion by wear. The results in Table 2 clearly show the existence of accelerated wear loss by corrosion. There is obvious synergistic effect between corrosion and wear, which leads to corrosion- induced wear and wear-induced corrosion. Efforts have been made by researchers to quantify such synergistic effect using the following approach [8,18-20]. The volume loss may be explained by defining the following terms:
Kwc=Kw+Kc (4)
where Kwc is the total volume loss owing to corrosion-wear, Kw is the material loss owing to wear, and Kc is the material loss owing to corrosion. Here, Kw can be divided into Kwo and △Kw:
Kw=Kwo+△Kw (5)
where Kwo is the pure wear loss in the absence of corrosion, and △Kw is the volume loss owing to the effect
of corrosion on wear. And Kc can be divided into Kco and △Kc:
Kc=Kco+△Kc (6)
where Kco is volume loss owing to corrosion in the absence of wear, and △Kc is volume loss owing to effect of wear on corrosion, or the enhancement of corrosion induced by wear. Moreover, the material loss due to synergistic effect between corrosion and wear (△K) can be expressed as
△K=△Kc+△Kw (7)
The material loss caused by corrosion Kc is given by Faraday equation as
Kc=Q/(ZF) (8)
Kc=MIt/(ZF) (9)
where Q is the charge passed, Z is the number of electrons involved in corrosion process, F is Faraday constant (96500 C/mol), M is the atomic mass of alloy and can be calculated by considering the mass fraction of components of the alloy, I is corrosion current, and t is corrosion time. Volume loss induced by wear in the absence of corrosion, Kwo, can be estimated by measuring volume change in cathodic condition at -0.8 V. As corrosion current cannot be directly measured at OCP, current density value is taken as corrosion current density calculating from polarization curve by standard Tafel procedure (Table 1).
The results of various contributions to material loss of Hastelloy C276 alloy in tribocorrosion are given in Table 3. The contribution of Kco to material loss is very small and can be negligible as evidenced from Table 3. It can be found that ratio of synergistic effect (△K) is in the range of 17.2%-22.4%, indicating that synergistic effect between corrosion and wear is significant. The ratio of △K/Kwc decreases slightly with increasing normal load and rotation speed, which means that synergistic effect is significant especially at low normal load and rotation speed. In addition, the contribution of pure wear to total material loss exceeds 70%, which means that pure wear is the dominant factor during corrosion-wear process.
Table 3 Contribution of corrosion and wear to material loss under different corrosion-wear conditions
Figure 5 shows the fractions of material loss components Kwo, △Kw and △Kc. It should be emphasized that although electrochemical dissolution is significantly accelerated and corrosion rate is increased by hundreds of times due to friction, the contribution of wear-induced-corrosion to total material loss (△Kc/Kwc) is not very high. Actually, the maximum value is 6.7% under 100 N and 100 r/min and the minimum value is only 2.5% under 200 N and 200 r/min. The ratio △Kc/Kwc decreases obviously with the increase of normal load and rotation speed. The ratio of the corrosion-induced-wear to total material loss (△Kw/Kwc) is high, which is in the range of 14.6%-20.5% under all experiment conditions. Corrosion-induced-wear obviously increases the material loss. It should be emphasized that contribution of wear-induced-corrosion and corrosion-induced-wear to synergism in tribocorrosion of several passive alloys has been studied. JIANG et al [18] found that contact frequency affects the synergistic effect between wear and corrosion. At high frequency, contact interval is sufficiently short and repassivation of the wear track is prevented, which makes the wear track always in active state, leading to a large contribution of wear-induced- corrosion to total material loss. While at low frequency, great contact interval time gives wear damaging through surface and subsurface crack initiation and propagation enough time to take place, leading to a large contribution of corrosion-induced-wear to synergism. The results obtained in this experiment show that under all experiment conditions, pure mechanical wear is dominant; for synergism between corrosion and wear, corrosion-wear-induced is dominant.
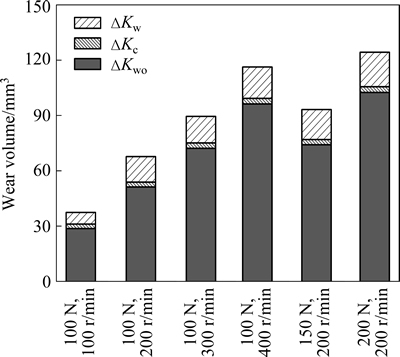
Fig. 5 Fractions of material loss factors under tribocorrosion of Hastelloy C276 alloy
Figure 6 shows the typical micrographs taken from wear tracks under OCP and cathodic potential conditions at 100 N and 200 r/min. It can be seen that worn surfaces at cathodic potential are characterized by mainly severe deformation regions interrupted by fine furrows parallel to the sliding direction, as shown in Fig. 6(a). Small cracks and thin flakes are also found on the worn surface. Under OCP condition, many wear debris are observed in Figs. 6(b) and (c), exhibiting many small corrosion pits inside the wear track at OCP. The corrosion current density is high inside the wear track during tribocorrosion, resulting in the formation of corrosion pits. And there are no apparent corrosion pits under cathodic potential condition.

Fig. 6 Typical micrographs taken from wear tracks for Hastelloy C276 alloy at 100 N and 200 r/min
Wear can highly accelerate corrosion current density due to the following reasons. 1) Passive film is damaged or removed by wear. Consequently, bare surfaces expose to electrolyte and suffer from large metal dissolution. 2) Wear causes plastic deformation in wear track and increases the densities of point defects, cracks and dislocations, which make active surface and high corrosion rate. The presence of corrosion pits shown in Fig. 6(c) is attributed to the local corrosion in active area with high electrochemical activity [2].
Corrosion-induced-wear can be understood through the failure mechanism of passive film. According to the model proposed by JIANG et al [18], wear in tribocorrosion can be treated as a low cycle fatigue process involving crack initiation and propagation. Figure 6(c) shows the micro cracks and fatigue cracks in wear track. The tip of crack has high-density of point defects and dislocations, which have high activity and prone to dissolution. Corrosion can promote the crack initiation and propagation on the worn surface. In addition, corrosion pits can serve as a site for crack nucleation and a site of stress concentration, which can also promote crack initiation and propagation.
4 Conclusions
1) The cathodic shift of open circuit potential of Hastelloy C276 alloy in artificial seawater under sliding contact with alumina pin due to friction is confirmed and current density is increased by three orders of magnitude as compared with that without sliding.
2) There is clear synergistic effect between corrosion and wear, which leads to corrosion-induced- wear and wear-induced-corrosion during tribocorrosion. Wear loss of Al2O3 pin is also large when it slides against Hastelloy C276 alloy and the surface is roughened.
3) The contribution of pure wear to total material loss exceeds 70% and pure wear is the dominant factor in corrosion-wear process. For considering synergism between corrosion and wear, the contribution of wear-induced-corrosion to total material loss is not very high although corrosion rate is greatly accelerated by friction. The fraction of corrosion-induced-wear is high and in the range of 14.6%-20.5% for all experiments.
References
[1] LANDOLT D, MISCHLER S, STEMP M. Electrochemical methods in tribocorrosion: A critical appraisal[J]. Electrochimica Acta, 2001, 46(24-25): 3913-3929.
[2] SUN Y, RANA V. Tribocorrosion behaviour of AISI 304 stainless steel in 0.5M NaCl solution[J]. Materials Chemistry and Physics, 2011, 129(1-2): 138-147.
[3] LANDOLT D. Electrochemical and materials aspects of tribocorrosion systems [J]. Journal of Physics D: Applied Physics, 2006, 39(15): 3121-3127.
[4] CHEN Jun, YAN Feng-yuan. Tribocorrosion behaviors of Ti-6Al-4V and Monel K500 alloys sliding against 316 stainless steel in artificial seawater [J]. Transactions of Nonferrous Metals Society of China 2012, 22(6): 1356-1365.
[5] MISCHLER S, MUN OZ A I. Wear of CoCrMo alloys used in metal-on-metal hip joints: A tribocorrosion appraisal [J]. Wear, 2013, 297(1-2): 1081-1094.
[6] ESPALLARGAS N, MISCHLER S. Tribocorrosion behaviour of overlay welded Ni-Cr 625 alloy in sulphuric and nitric acids: Electrochemical and chemical effects [J]. Tribology International, 2010, 43(7): 1209-1217.
[7] BI Qing-lin, LIU Wei-min, MA Ji-qiang, YANG Jun, PU Yu-ping, XUE Qun-ji. Tribocorrosion behavior of Ni-17.5Si-29.3Cr alloy in sulfuric acid solution [J]. Tribology International, 2009, 42(7): 1081-1087.
[8] IWABUCHI A, LEE J W, UCHIDATE M. Synergistic effect of fretting wear and sliding wear of Co-alloy and Ti-alloy in Hanks' solution [J]. Wear, 2007, 263(1-6): 492-500.
[9] PONTHIAUX P, WENGER F, DREES D, CELIS J P. Electrochemical techniques for studying tribocorrosion processes [J]. Wear, 2004, 256(5): 459-468.
[10] DIOMIDIS N, CELIS J P, PONTHIAUX P, WENGER F. Tribocorrosion of stainless steel in sulfuric acid: Identification of corrosion-wear components and effect of contact area[J]. Wear, 2010, 269(1-2): 93-103.
[11] TEKIN K C, MALAYOGLU U. Assessing the tribocorrosion performance of three different nickel-based superalloys [J]. Tribology Letters, 2010, 37(3): 563-572.
[12] SALASI M, STACHOWIAK G B, STACHOWIAK G W. New experimental rig to investigate abrasive-corrosive characteristics of metals in aqueous media [J]. Tribology Letters, 2010, 40(1): 71-84.
[13] BURSTEIN G T, MARSHALL P I. Growth of passivating films on scratched 304L stainless steel in alkaline solution [J]. Corrosion Science, 1983, 23(2): 125-137.
[14] STACK M M. Mapping tribo-corrosion processes in dry and in aqueous conditions: Some new directions for the new millennium [J]. Tribology International, 2002, 35(10): 681-689.
[15] CHEN Jun, YAN Feng-yuan, CHEN Bei-bei, WANG Jian-zhuang. Assessing the tribocorrosion performance of Ti6Al4V, 316 stainless steel and Monel K500 alloys in artificial seawater [J]. Materials and Corrosion, 2013, 64(5): 394-401.
[16] AKONKO S, LI D Y, ZIOMEK-MOROZ M. Effects of cathodic protection on corrosive wear of 304 stainless steel [J]. Tribology Letters, 2005, 18(3): 405-410.
[17] IWABUCHI A, SONODA T, YASHIRO H, SHIMIZU T. Application of potential pulse method to the corrosion behavior of the fresh surface formed by scratching and sliding in corrosive wear [J]. Wear, 1999, 225-229(Part 1): 181-189.
[18] JIANG J, STACK M M, NEVILLE A. Modelling the tribo-corrosion interaction in aqueous sliding conditions [J]. Tribology International, 2002, 35(10): 669-679.
[19] WATSON S W, FRIEDERSDORF F J, MADSEN B W, CRAMER S D. Methods of measuring wear corrosion synergism [J]. Wear, 1995, 181: 476-484.
[20] CHEN Jun, YAN Feng-yuan. Corrosive wear performance of Monel K500 alloy in artificial seawater [J]. Tribology Transactions, 2013, 56(5): 848-856.
Hastelloy C276合金在模拟海水环境中的腐蚀磨损协同作用
陈 君1,2,3,王建章2,阎逢元2,张 清1,3,李全安1,3
1. 河南科技大学 材料科学与工程学院,洛阳 471023;
2. 中国科学院 兰州化学物理研究所 固体润滑国家重点实验室,兰州 730000;
3. 有色金属共性技术 河南省协同创新中心,洛阳 471023
摘 要:利用带有电化学测试体系的销-盘腐蚀磨损试验机系统研究Hastelloy C276 合金在模拟海水中与Al2O3 陶瓷对磨时的腐蚀和腐蚀磨损行为。结果表明,摩擦作用不仅使Hastelloy C276合金的开路电位大幅下降,而且将合金的腐蚀电流密度提高了3个数量级。Hastelloy C276 合金的磨蚀与磨损之间存在明显的协同作用,磨损促进了腐蚀,反过来腐蚀也加速了磨损,在本实验摩擦条件下纯机械磨损在金属总磨损量中的占比均超过70%,这说明机械磨损作用是合金腐蚀磨损失效的主导因素。虽然摩擦使得Hastelloy C276合金的腐蚀速度得到极大提高,但是磨损对腐蚀的促进量在金属总磨损量中的占比并不大,而腐蚀对磨损的促进量在总磨损量中的占比较高,在14.6%~20.5%范围内变化。
关键词:腐蚀磨损;Hastelloy C276 合金;协同作用;海水
(Edited by Wei-ping CHEN)
Foundation item: Project (LSL-1310) supported by the Open Project of State Key Laboratory of Solid Lubrication, China; Project (51171059) supported by the National Natural Science Foundation of China
Corresponding author: Jun CHEN; Tel: +86-15225508091; E-mail: chenjun318822200@163.com
DOI: 10.1016/S1003-6326(15)63650-0
Abstract: A systematic investigation was carried out to discuss the corrosion and tribocorrosion behaviors of Hastelloy C276 alloy sliding against Al2O3 pin in artificial seawater, using a pin-on-disk tribometer integrated with a potentiostat for electrochemical control. The results show that the great decrease of open circuit potential and three orders of magnitude increase of corrosion current density occur caused by friction. There are clearly synergistic effect between corrosion and wear, resulting in corrosion-induced-wear and wear-induced-corrosion in tribocorrosion process. The contribution of pure mechanical wear to total material loss exceeds 70% in all sliding conditions, so mechanical wear is the dominant factor during tribocorrosion. For considering synergistic effect between corrosion and wear, the contribution of wear-induced-corrosion to total material loss is not very high although corrosion rate is greatly accelerated by friction. The fraction of corrosion-induced-wear to the total material loss is high and in the range of 14.6%-20.5% under all sliding conditions.


