文章编号:1004-0609(2015)10-2936-09
嗜酸铁氧化富集物高效浸提废覆铜板分选残渣中的铜
仉丽娟1,刘晓文2,康 鑫1,陈岩贽2,周文根1,史美玉1,周文博1,
温 勇2,王 炜2,周洪波1, 2
(1. 中南大学 资源加工与生物工程学院 生物冶金教育部重点实验室,长沙 410083;
2. 环境保护部 华南环境科学研究所,广州 510655)
摘 要:
利用生物浸出的方法实现废覆铜板分选渣中残留铜的资源化,主要研究Fe2+物质添加量、浸出时间、初始pH和渣投加量(固形物含量)等因素对不同来源废覆铜板渣中铜生物浸出的影响。结果表明:生物浸出铜过程中无需额外再添加Fe2+能源物质且能够短时间内(≤5 h)快速高效浸出不同来源分选残渣中的铜;初始pH和渣投加量对废覆铜板渣中铜浸出产生显著影响。优化结果表明:控制初始pH≤2.2,渣投加量20%~30%,无额外添加酸和Fe2+,两种分选残渣生物浸出5 h后,铜浸出率可达95%以上。
关键词:
中图分类号:Q939.97 文献标志码:A
High extraction of copper from flotation tailings of waste copper-clad laminates by acidophilic iron-oxidizing enrichment
ZHANG Li-juan1, LIU Xiao-wen2, KANG Xin1, CHEN Yan-zhi2, ZHOU Wen-gen1, SHI Mei-yu1,
ZHOU Wen-bo1, WEN Yong2, WANG Wei2, ZHOU Hong-bo1, 2
(1. Key Laboratory of Biometallurgy, Ministry of Education,
School of Minerals Processing and Bioengineering, Central South University, Changsha 410083, China;
2. South China Institute of Environmental Sciences, Ministry of Environment Protection, Guangzhou 510655, China)
Abstract: Recycling of the flotation tailings of waste copper-clad laminates was achieved by bioleaching method, the effects of Fe2+ re-addition, leaching time, initial pH and pulp density on the copper extraction were investigated. The results show that an effective copper extraction can be achieved without re-addition of Fe2+ within 5 h. The initial pH and pulp density play important roles in copper extraction. Under the optimized condition of initial pH≤2.2 and pulp density 20%-30%, the Cu leaching rate is more than 95% in 5 h without re-addition of Fe2+ and acid.
Key words: waste copper-clad laminate; flotation tailings; bioleaching; copper recycling; acidophilic iron-oxidizing enrichment
2010年我国的覆铜板制造总量已达到3.99×109 m2,大约占全球覆铜板制造总量的80%。然而,按照覆铜板制造和加工成印刷电路板过程中约20%材料废品率计算,将伴随年产量约为0.8×109 m2覆铜板边角料(废覆铜板)的产生。
废覆铜板中铜含量不同,质量分数低的约为15%,高的可大于70%,因而其资源化再生价值极高,可作为一种重要的铜回收再利用的“城市矿产资源”[1]。
目前废覆铜板中金属铜回收主要通过破碎、粉碎、摇床分选等一系列技术流程获得覆铜板中的铜粉[1-4]。但是,由于该工艺的技术限制性,导致浮选后产生大量的残渣,仅广东清远地区每年的残渣产生量就多达4×104 t。该部分浮选残渣中残留0.5%~1%铜(质量分数),与我国的铜矿平均品位0.8%相近,达到了再利用的程度。大量废覆铜板浮选残渣不仅占用大量的土地,而且余留的金属铜及大量的树脂、纤维及溴代阻燃剂等有害物质可通过各种方式加速释放到环境中,将对我们的生存环境和人体健康构成严重的危害[5]。因而实现该类固体废物的“资源化、减量化、无害化”,最终实现环境与经济发展的“共赢”具有重要意义[6]。
针对各种电子废物的处理的方法有化学冶金、火法冶炼、电解、燃烧、热解、超临界流体及机械-物理等[3, 7-11]。这些方法不能有效分离低品位固废(如本研究的废覆铜板渣)中有毒金属,且消耗大量的酸及能源,还易造成其他有毒有害物质的挥发、扩散,形成二次污染[12]。生物浸提法作为近年来在生物冶金的基础上发展起来的资源化利用新技术,在废印刷线路板、废旧电池、城市污泥等资源化、无害化处理中逐渐受到关注[2, 13-15]。生物冶金(Bioleaching)是利用微生物的氧化、酸溶、代谢产物螯合等作用使矿物中的金属溶解,从而达到浸提金属目的的技术[16]。该技术具有成本低、环境友好等特点,已经被应用于Cu、Co、Ni、Zn、U等难溶金属硫化物(或其氧化物)的浸出,或用以去除阻碍与金属接触的表面杂质[17-18]。然而,目前还没有生物浸出技术用于处理废覆铜板分选残渣中残留铜的报道。
本文作者通过与传统化学酸浸进行对比,考察了经长期驯化获得的嗜酸铁氧化富集物分别浸提两种不同废覆铜板残渣中铜(企业厂区内部和野外长期堆放)的可行性,并着重考察了Fe2+添加、浸出时间、初始pH和渣投加量(固形物含量)等因素对生物浸出残渣中铜的影响。
1 实验
1.1 菌种及培养条件
将采自不同矿堆或煤堆废水、温泉水样,以及本实验室经黄铜矿长期驯化获得中等高温嗜酸微生物富集物[19]混合,在室温(30 °C)、初始pH 1.8、44.7 g/L FeSO4·7H2O条件下,添加2%(质量分数)的废覆铜板残渣于2 L搅拌槽中进行连续富集培养、驯化,获得了以Acidithiobacillus ferrooxidans和Leptospirillum ferriphilum等为主的嗜酸铁氧化微生物富集物。该富集物将用于后续废覆铜板残渣生物浸出实验。
1.2 废覆铜板浮选残渣
实验所用的两种废覆铜板浮选残渣来自广东清远市,包括企业厂区内堆放的湿法浮选后废覆铜板渣(未长期堆放)和长期堆放于野外的废覆铜板浮选渣。在生物浸出前,将废覆铜板浮选渣首先通过孔径为380 μm筛除去石子等大颗粒物质,然后进一步破碎,干式筛选粒径≤0.75 μm渣粒。ICP分析金属元素含量指出:企业堆渣中含0.81%Cu和0.27%Fe(质量分数);野外堆渣中含0.75%Cu和1.87%Fe。由于元素Cu在废覆铜板残渣中含量低(<1%)及渣成分的复杂性,X射线衍射未能检测到铜在残渣中的物相形式。但是依据覆铜板性质[20],无论企业内堆放的还是野外长期堆放的废覆铜板残渣中铜主要以金属铜或氧化铜的形式存在。
1.3 试验方法
根据生物冶金浸出机理[18, 21],Fe2+添加量、浸出时间、初始pH和固形物含量(废覆铜板渣投加量)等因素显著影响金属的浸出。因此着重研究上述因素对废覆铜板残渣中铜生物浸出效果的影响,如表1所列。
所有浸出实验于500 mL摇瓶中进行,按5%的接种量将上述嗜酸铁氧化微生物富集物菌液(终浓度约为1×107 mL-1)接种于灭菌的190 mL的9 K营养盐培养基[22]中,同时加入2%的废覆铜板残渣,培养7~10 d至体系变红,同时pH降至2.0±0.05左右,此时加入废覆铜板残渣开始生物浸出[23]。在浸出阶段分别考察了Fe2+能源再添加、浸出时间、浸出初始pH和废覆铜板残渣投加量等不同因素(见表1)对铜浸出的影响。每组实验分别设立3个重复实验。
生物浸出废覆铜板残渣过程中,定时取样检测。样品经4 000 r/min离心3 min,然后将上清液滤过0.45 μm滤膜,最后收集滤液于无菌的EP管中,用于Cu等金属离子浓度检测。
表1 影响废覆铜板浮选残渣生物浸出的因素及水平
Table 1 Investigation of different factors and levels influencing copper extraction
1.4 分析方法
浸出液中可溶性铜含量采用原子吸收法检测。实验过程中浸出液氧化还原电位(ORP,oxidation reduction potential)的测定采用铂电极,Ag/AgCl电极作为参比电极;pH采用pH复合电极来测定。微生物数量通过血球计数板直接计数。
2 结果与讨论
2.1 生物浸提废覆铜板浮选残渣中铜机理
生物浸提废覆铜板浮选残渣中金属铜或氧化铜主要机理如式(1)~(5)所示。本研究中铁氧化嗜酸微生物富集物主要是依靠细菌浸出的间接作用[23-25]浸提废覆铜板浮选渣中的铜,即该富集物能快速将Fe2+氧化成Fe3+(见式(1)),加速铜浸出剂Fe3+生成,而生物转化的高铁Fe3+将单质铜氧化(见式(2)),生成的Cu2+溶于酸性浸出液中。此外,Fe3+大量存在易形成沉淀且伴随H+产生(见式(3)~(4)),H+促进了氧化铜的溶解(见式(5))。因而,废覆铜板浮选残渣中残留铜的浸出速率主要由生物铁生成(Fe3+与Fe2+摩尔比)和化学反应速率决定。而亚铁能源、浸出pH、固形物含量及时间对生物铁生成和化学反应速率影响显著。

2Fe3++Cu0→2Fe2++Cu2+ (2)
Fe3++3H2O→Fe(OH)3↓+3H+ (3)
6Fe3++12H2O+2A++4SO42-→2AFe3(SO4)2(OH)6↓+12H+ (4)
CuO+2H+→Cu2++H2O (5)
式(4)中:A表示K+、Na+、NH4+等单价离子。
2.2 Fe2+再添加对废覆铜板浮选残渣中铜浸出的影响
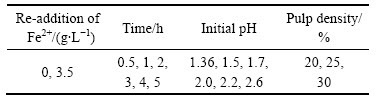
图1和2所示为浸出过程中Fe2+能源再添加对不同来源的废覆铜板残渣生物浸出的影响。其中,图1(a)和2(a)分别显示了Fe2+能源再添加对来源于企业内堆放的和野外长期堆放的废覆铜板残渣中铜浸出的影响。结果表明:浸出2 h左右,两种生物浸出实验组中(添加Fe2+标记为“Bacteria+Fe2+”;和不添加Fe2+标记为“Bacteria”),铜浸出率相近且均达到95%以上,浸出持续至5 h后,废渣中的铜被浸出完全。由此可知,亚铁能源再添加对铜浸出率没有影响,主要归因于嗜酸铁氧化菌培养过程中富集大量铁氧化菌,同时氧化亚铁生成大量生物高铁Fe3+;浸出过程中生物高铁Fe3+会氧化铜而被还原成Fe2+,而Fe2+被体系中大量存在的铁氧化菌快速转化成Fe3+,因而该生物浸出过程能够持续循环进行。
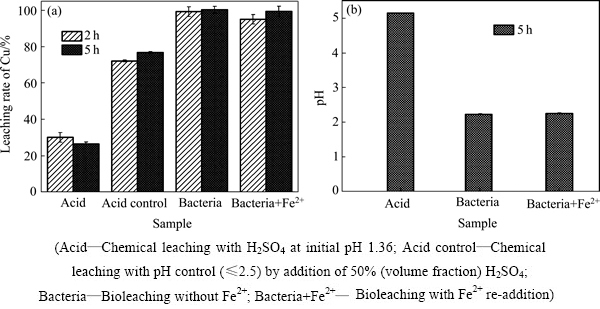
图1 在初始pH 1.36、某企业内堆放的废覆铜板渣添加量20%条件下Fe2+添加对生物浸提废覆铜板渣中铜浸出率和pH的影响
Fig. 1 Effect of Fe2+ re-addition on copper leaching rate(a) and pH(b) in different flasks with initial pH 1.36 and 20% pulp density of floatation tailings stored in some enterprise
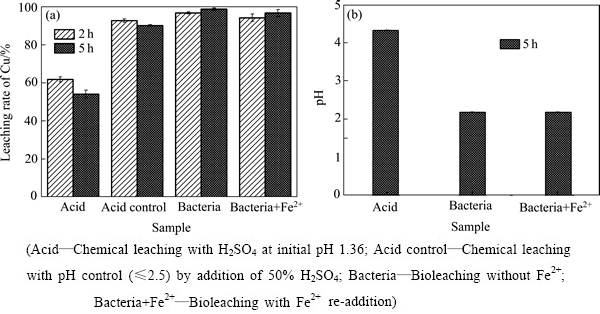
图2 在初始pH 1.36、野外长期堆放的废覆铜板渣添加量20%条件下Fe2+添加对生物浸提废覆铜板渣中铜浸出率和pH的影响
Fig. 2 Effect of Fe2+ addition on copper leaching rate(a) and pH(b) in different flasks with initial pH 1.36 and 20% pulp density of floatation tailings stored long in wild
由图1(a)可知,微生物浸出废覆铜板残渣中铜的效果明显优于化学酸浸的。同等初始pH(pH=1.36)的化学酸浸实验中(未加菌液和Fe2+能源,同时未施加酸调控pH,标记为“Acid”),浸出2 h和5 h后,铜浸出率分别为30.2%和26.6%(企业内堆渣)和61.8%和54.1%(野外堆渣);其中,浸出5 h的浸出率较低,主要归因于废覆铜板残渣溶解不断耗酸,导致浸出液的pH偏高,pH>4(见图1(b)和2(b)),因而浸出的Cu2+水解再次被沉淀析出(Cu2++2H2O→Cu(OH)2↓+2H+)。而酸调控化学浸出(pH≤2.5,标记为“Acid control”)实验组中,铜浸出率显著提高,企业内废覆铜板渣中铜浸出率分别为72.2%(2 h)和76.9%(5 h),野外堆渣中铜浸出率为90%~92%,但仍然低于两组生物浸出实验组的(见图1(a)和2(a))。尤其值得注意的是,生物浸出实验组(初始pH=1.36)浸出过程中无额外添加酸调控体系pH,浸出5 h后,生物浸出组pH不超过2.5(见图1(b)和2(b))。因而,与酸浸相比,生物浸出废覆铜板渣中金属铜速率快,效率高,且无需外加酸和Fe2+能源,大大降低了酸耗和工艺成本。
2.3 浸出时间对废覆铜板浮选渣中铜浸出的影响
浸出时间反应了废覆铜板残渣湿法浸出过程的速率。废覆铜板残渣中铜无害化资源化处理所需时间越短,表明湿法工艺速率越快,则实际工业应用越有可行性。图3和4所示分别为在酸浸或生物浸出过程中企业内堆放和野外堆放的废覆铜板渣的铜浸出率、pH和氧化还原电位ORP随时间的变化。
从图3(a)和4(a)铜浸出变化结果可以看出:无论是企业堆渣,还是野外堆渣,生物浸出过程均能快速高效浸提废覆铜板残渣中的铜。当生物浸出1 h,堆渣中的95%以上的铜被浸出,且此后浸出液中的铜一直稳定存在。与生物浸出过程相反,酸浸过程的铜浸出率不断降低,归因于堆渣中存在其他碱性物质不断耗酸导致体系pH上升(见图3(b)和4(b)),进而使得浸出的铜再次沉淀析出。从图3(c)和4(c)可以看出,生物浸出能够有效维持体系的高ORP环境,促进了金属铜的浸出,并维持浸出Cu2+在溶液中的稳定存在。
2.4 初始pH对废覆铜板浮选残渣中铜浸出的影响
浸出体系pH影响废覆铜板渣中铜浸出效率及溶出的金属离子在溶液中的存在。因此,研究不同初始pH对废渣中铜浸出、体系pH和ORP的影响,结果如图5和6所示。
图3 在初始pH 1.5,企业内堆放的废覆铜板渣添加量20%条件下生物浸出和化学酸浸废覆铜板渣实验中铜浸出率、pH和ORP随时间变化
Fig. 3 Variations of copper leaching rate(a), pH(b) and ORP(c) with time in different experiments at initial pH 1.5 and 20% pulp density of floatation tailings stored in some enterprise
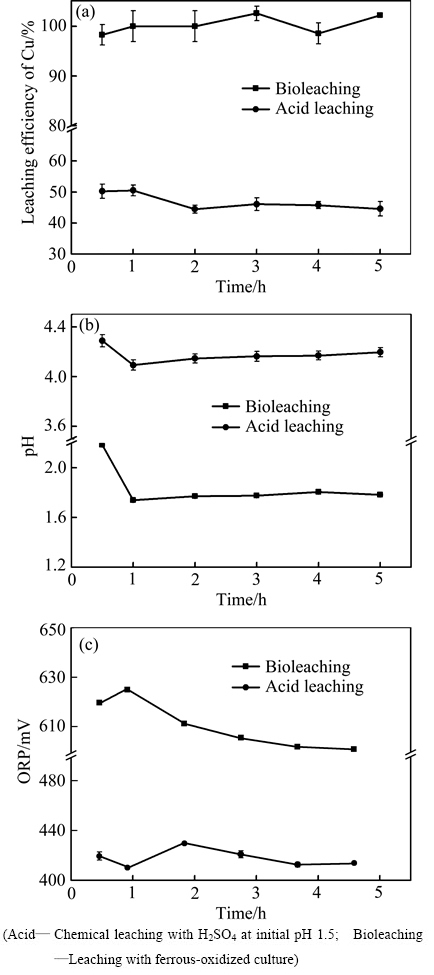
图4 在初始pH 1.5,野外堆放的废覆铜板渣添加量20%条件下生物浸出和化学酸浸废覆铜板渣实验中铜浸出率、pH和ORP随时间变化
Fig. 4 Variations of copper leaching rate(a), pH(b) and ORP(c) with time in different experiments at initial pH 1.5 and 20% pulp density of flotation tailings stored long in wild
由图5(a)可知,化学酸浸或生物浸出体系中,不同初始pH对企业堆渣中铜浸出产生显著的影响。在化学酸浸实验中,初始pH设为1.36或2.0时,浸出5 h后,体系pH均升高且超过5.0,且此时体系氧化还原电位(ORP小于300 mV)低于金属铜氧化标准电极电位(φΘ(Cu2+/Cu)=337 mV),如图5(b)所示。pH影响金属离子在溶液中的存在;而依据Nernst方程,体系氧化还原电位(ORP)与Fe3+与Fe2+摩尔比呈现正比关系[26]。因而根据2.1节的浸出机理,高pH值和低ORP不利于废渣中铜溶出,与本研究中铜化学酸浸结果一致,即不同初始pH纯化学酸浸下铜最终浸出率不高于30%(5 h),尤其是在初始pH 2.0酸浸条件下,铜几乎未被浸出(见图5(a))。与酸浸实验组相比,生物浸出显著促进废覆铜板残渣中金属铜的浸出,但是仍受到初始pH的影响。由图5(a)可知,当初始pH由1.36增加至2.2,不同生物浸出实验组中铜初始浸出率(2 h)随初始pH的升高而略有降低,但延长浸出时间至5 h后,铜浸出率进一步提高,并最终相近(≥98%),且此时所有体系pH维持在2.2~2.3之间,ORP约为480 mV(见图5(c)),均显著高于金属铜氧化标准电极电位(φΘ(Cu2+/Cu)=337 mV),高ORP也表明体系存在足够的Fe3+离子[26]。然而,当初始pH增加至2.6,铜浸出率显著降低,浸出2 h和5 h时铜浸出率分别仅有74.0%和83.1%;较高pH(2.55)和较低ORP(438 mV)(见图5(b)和(c))的浸出环境与较低铜浸出率相符。
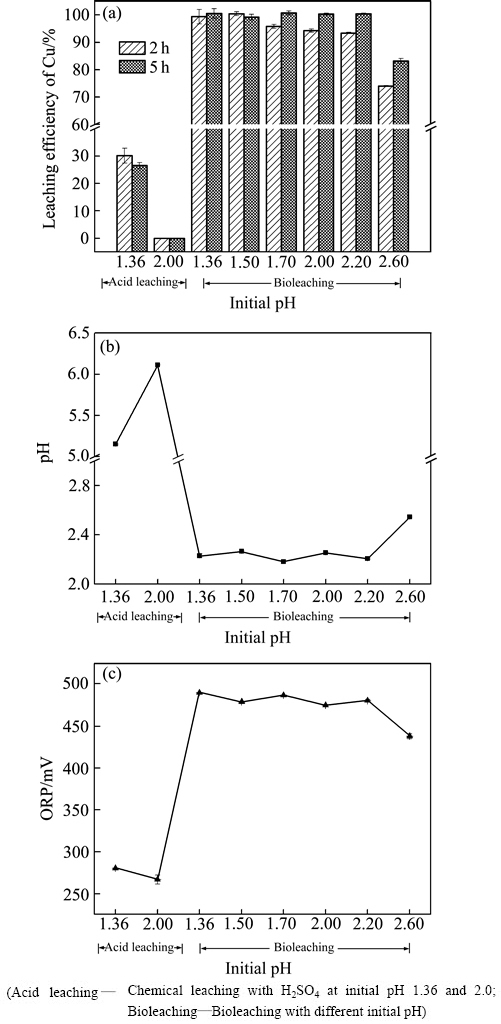
图5 某企业废覆铜板渣添加量20%条件下不同初始pH对酸浸和生物浸出废覆铜板渣中铜浸出率、pH和ORP的影响
Fig. 5 Effects of initial pH on copper leaching rate(a), pH(b) and ORP(c) in different experiments with 20% pulp density of flotation tailings inside some company
与企业堆渣生物浸出结果相似,图6所示为不同初始pH对野外堆放的废覆铜板残渣生物或化学酸浸出体系的影响。在化学酸浸中,初始pH对废覆铜板中铜浸出产生显著的影响。尤其是初始pH为2.0时,浸出5 h时体系pH升高至6.02,铜浸出率为0,显著低于初始pH为1.36的酸浸组(54.1%,5 h)。生物浸出实验组中,当初始pH由1.36增加至1.7时,对体系pH、ORP和铜浸出率没有显著影响;进一步提高初始pH至2.2,铜浸出率略有降低,但最终铜浸出率仍达到96%;然而当初始pH升高至2.6时,铜浸出率显著降低,分别为77.8%(2 h)和87.1%(5 h)。

图6 野外堆放的废覆铜板渣添加量20%条件下不同初始pH对酸浸和生物浸出废覆铜板渣中金属铜浸出率、pH和ORP的影响
Fig. 6 Effects of initial pH on copper leaching rate(a), pH(b) and ORP(c) in different experiments with 20% pulp density of flotation tailings stored in wild
由图5和6可知,初始pH对生物浸出废覆铜板渣中铜有显著影响,但控制初始pH≤2.2,在20%覆铜板渣添加量的条件下,均能实现高效快速的铜浸出。
2.5 固形物含量对废覆铜板浮选渣中铜浸出的影响
覆铜板分选残渣中占99%左右的都是非金属成分,致使渣样密度低。在浸出过程中,堆渣添加量过高容易出现样结团漂浮,尤其是企业堆渣。因而,本研究结合覆铜板渣固有特性,在初始pH 2.0的条件下,分别研究了20%,25%和30% 3个可行的高含固率对废覆铜板渣生物浸出的影响。
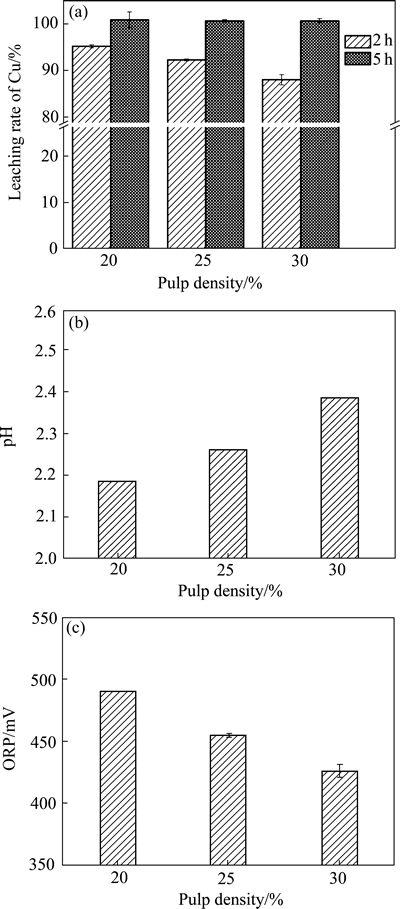
图7 不同渣投加量生物浸出企业内废覆铜板渣中铜出率、pH和ORP的影响
Fig. 7 Effects of pulp density of flotation tailings from company on copper leaching rate(a), pH(b) and ORP(c) in different experiments with initial pH 2.0
图7和8所示分别为不同废覆铜板残渣添加量对生物浸出体系的影响。结果显示:两种废覆铜板分选残渣生物浸出过程中,当渣投加量由20%增加至25%和30%、浸出2 h时铜浸出率降低,分别为95.3%,92.3%和80.1%(企业内堆渣)和99%,98.3%和96.9%(野外长期堆渣)(见图7(a)和8(a))。但是延长浸出时间至5 h后,3种高投加量的体系中均能实现近100%铜高效浸出。渣投加量越高,体系内耗酸物质愈多,因而体系终点浸出pH值越高,但仍不超过2.5(见图7(b)和8(b));伴随渣投加量的增加,体系渣中含铜量越高,因而耗Fe3+越多,最终体系ORP越低(见图7(c)和8(c))。
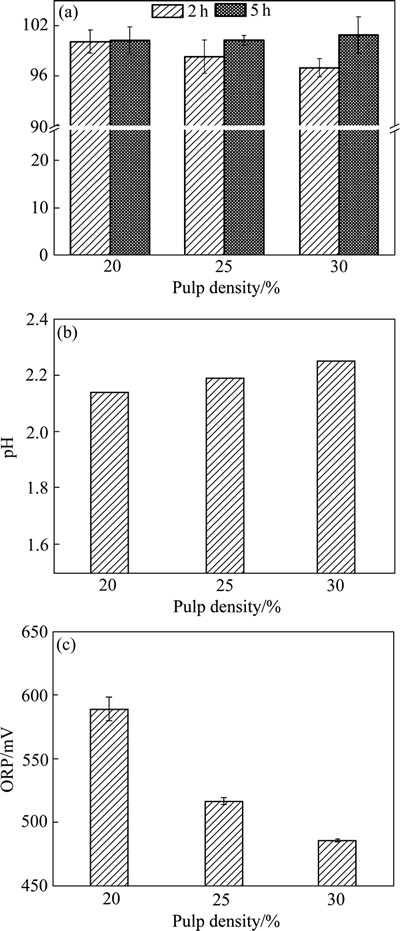
图8 不同渣投加量对生物浸出野外废覆铜板渣中铜浸出率、pH和ORP的影响
Fig. 8 Effects of pulp density of flotation tailings stored long in wild on copper leaching rate(a), pH(b) and ORP(c) in different experiments with initial pH 2.0
3 结论
1) 相比于化学酸浸或酸调控浸出,嗜酸铁氧化富集物生物浸出废覆铜板残渣中的铜更具有可行性,即生物浸出过程不仅提高了废覆铜板渣中铜浸出速率和最终浸出率,而且降低了酸耗,其浸出过程中无需外加酸和Fe2+能源物质。
2) 生物浸出能够短时间内(≤5 h)快速高效浸出废覆铜板残渣中的铜,浸出率达95%以上。
3) 生物浸出初始pH显著影响覆铜板渣中铜浸出。控制浸出初始pH≤2.2,能够有效地提高铜浸出率。
4) 高覆铜板投加量(20%~30%)对生物浸出铜不利,尤其是企业堆渣,但延长浸出时间,铜浸出率进一步提高,5 h后被完全浸出。
5) 本研究获得的嗜酸铁氧化微生物富集物能够高效快速浸出高投加量的废覆铜板残渣中的铜,因而生物处理该类废覆铜板渣具有巨大潜力。
REFERENCES
[1] 项 赟, 杜建伟, 温 勇, 雷伟香. 废覆铜板湿法分选残渣资源化利用技术[C]//第二届重金属污染防治技术及风险评价研讨会暨重金属污染防治专业委会2012年首届学术年会, 2012: 456-461.
XIANG Yun, DU Jian-wei, WEN Yong, LEI Wei-xiang. Utilization technology of flotation tailings of waste copper-clad plate[C]// The 2th Assessment of Heavy Metal Pollution Prevention And Control Technology and Assessment, and the First Annual Meeting of Heavy Metal Pollution Prevention Professional Committee, 2012: 456-461.
[2] ZHU N, XIANG Y, ZHANG T, WU P, DANG Z, LI P, WU J. Bioleaching of metal concentrates of waste printed circuit boards by mixed culture of acidophilic bacteria[J]. Journal of Hazardous Materials, 2011, 192(2): 614-619.
[3] HUANG K, GUO J, XU Z. Recycling of waste printed circuit boards: A review of current technologies and treatment status in China[J]. Journal of Hazardous Materials, 2009, 164(2): 399-408.
[4] 顾帼华, 戚云峰. 废旧印刷电路板的粉碎性能及资源特征[J]. 中国有色金属学报, 2004, 14(6): 1037-1041.
GU Guo-hua, QI Yun-feng. Crushing performance and resource characteristic of printed circuit board scrap[J]. The Chinese Journal of Nonferrous Metals, 2004, 14(6): 1037-1041.
[5] 袁剑刚, 郑 晶, 陈森林, 李 晶, 李 涵, 肖 迪, 余晓华, 罗 勇, 严 骁, 杨中艺, 罗孝俊, 麦碧娴. 中国电子废物处理处置典型地区污染调查及环境、生态和健康风险研究进展[J]. 生态毒理学报, 2013, 8(4): 473-486.
YUAN Jian-gang, ZHENG Jin, CHEN Sen-lin, LI Jin, LI Han, XIAO Di, YU Xiao-hua, LUO Yon, YAN Xia, YANG Zhong-yi, LUO Xiao-jun, MAI Bi-xian. Advances in the researches on investigations of pollution and environmental, ecological and health risks from E-waste recycling activities in China[J]. Asian Journal of Ecotoxicology, 2013, 8(4): 473-486.
[6] 王成彦, 邱定蕃, 徐盛明. 金属二次资源循环利用意义, 现状及亟需关注的几个领域[J]. 中国有色金属学报, 2008, 18(1): 359-366.
WANG Chen-yan, QIU Ding-pan, XU Sheng-ming. Significance, status and important issues for recycling utilization of metal secondary resource in China[J]. The Chinese Journal of Nonferrous Metals, 2008, 18(1): 359-366.
[7] ZHU P, CHEN Y, WANG L, ZHOU M. Treatment of waste printed circuit board by green solvent using ionic liquid[J]. Waste Management, 2012, 32(10): 1914-1918.
[8] FOGARASI S, IMRE-LUCACI F, IMRE-LUCACI A, ILEA P. Copper recovery and gold enrichment from waste printed circuit boards by mediated electrochemical oxidation[J]. Journal of Hazardous Materials, 2014, 273: 215-221.
[9] HUANG J, CHEN M, CHEN H, CHEN S, SUN Q. Leaching behavior of copper from waste printed circuit boards with Bronsted acidic ionic liquid[J]. Waste Management, 2014, 34(2): 483-488.
[10] HA V H, LEE J C, HUYNH T H, JEONG J, PANDEY B D. Optimizing the thiosulfate leaching of gold from printed circuit boards of discarded mobile phone[J]. Hydrometallurgy, 2014, 149: 118-126.
[11] 龙来寿, 孙水裕, 钟 胜, 刘敬勇, 邓 丰, 李红军. 真空热解预处理对回收废线路板中铜的影响[J]. 中国有色金属学报, 2010, 20(4): 795-800.
LONG Lai-shou, SUN Shui-yu, ZHONG Sheng, LIU Jing-yong, DENG Feng, LI Hong-jun. Effect of vacuum pyrolysis pretreatment on recovery of copper from scrap printed circuit boards[J]. The Chinese Journal of Nonferrous Metals, 2010, 20(4): 795-800.
[12] ORTUNO N, CONESA J A, MOLTO J, FONT R. Pollutant emissions during pyrolysis and combustion of waste printed circuit boards, before and after metal removal[J]. Science of the Total Environment, 2014, 499: 27-35.
[13] XIANG Y, WU P, ZHU N, ZHANG T, LIU W, WU J, LI P. Bioleaching of copper from waste printed circuit boards by bacterial consortium enriched from acid mine drainage[J]. Journal of Hazardous Materials, 2010, 184(1): 812-818.
[14] YANG Y, CHEN S, LI S, CHEN M, CHEN H, LIU B. Bioleaching waste printed circuit boards by Acidithiobacillus ferrooxidans and its kinetics aspect[J]. Journal of Hazardous Materials, 2014, 173: 24-30.
[15] 徐 颖, 谢志钢, 薛 璐, 冯岳阳, 陈 玉, 孙 瑛. 氧化亚铁硫杆菌淋滤重金属污染底泥的动力学[J]. 中国有色金属学报, 2014, 24(5): 1352-1358.
XU Ying, XIE Zhi-gang, XUE Lu, FENG Yue-yang, CHEN Yu, SUN Ying. Bioleaching kinetics of heavy metal from contaminated sediment by Thiobacillus ferrooxidans[J]. The Chinese Journal of Nonferrous Metals, 2014, 24(5): 1352-1358.
[16] VERA M, SCHIPPERS A, SAND W. Progress in bioleaching: fundamentals and mechanisms of bacterial metal sulfide oxidation-part A[J]. Applied Microbiology and Biotechnology, 2013, 97: 7529-7541.
[17] OLSON G, BRIERLEY J, BRIERLEY C. Bioleaching review part B: Progress in bioleaching: Applications of microbial processes by the minerals industries[J]. Applied Microbiology and Biotechnology, 2003, 63(3): 249-257.
[18] ROHWERDER T, GEHRKE T, KINZLER K, SAND W. Bioleaching review part A: Progress in bioleaching: fundamentals and mechanisms of bacterial metal sulfide oxidation[J]. Applied Microbiology and Biotechnology, 2003, 63(3): 239-248.
[19] WANG Y, ZENG W, CHEN Z, SU L, ZHANG L, WAN L, QIU G, CHEN X, ZHOU H. Bioleaching of chalcopyrite by a moderately thermophilic culture at different conditions and community dynamics of planktonic and attached populations[J]. Hydrometallurgy, 2014, 147/148: 13-19.
[20] LEE C M, LIM J H, HWANG S M, PARK E C, SHIM J H, PARK J H, JOO J, JUNG S B. Characterization of flexible copper laminates fabricated by Cu electroplating process[J]. Transactions of Nonferrous Metals Society of China, 2009, 19(4): 965-969.
[21] SAND W, GEHRKE T, JOZSA P. (Bio)chemistry of bacterial leaching-direct vs. indirect bioleaching[J]. Hydrometallurgy, 2001, 59: 159-175.
[22] SILVERMAN M P, LUNDGREN D G. Studies on the chemoautotrophic iron bacterium Ferrobacillus ferrooxidans: I. An improved medium and a harvesting procedure for securing high cell yields[J]. Journal of Bacteriology, 1959, 77(5): 642-647.
[23] XIN B, ZHANG D, ZHANG X, XIA Y, WU F, CHEN S, LI L. Bioleaching mechanism of Co and Li from spent lithium-ion battery by the mixed culture of acidophilic sulfur-oxidizing and iron-oxidizing bacteria[J]. Bioresource Technology, 2009, 100(24): 6163-6169.
[24] 曾伟民, 朱海珍, 叶子捷, 夏明晨, 喻子凌, 王新天. 生物湿法冶金技术回收废弃线路板中有价金属的研究进展[J]. 有色金属科学与工程, 2013, 4(1): 26-30.
ZENG Wei-min, ZHU Hai-zhen, YE Zi-jie, XIA Ming-chen,YU Zi-ling, WANG Xin-tian. Review on recovering precious metals from wasted circuit board by bio-hydrometallurgy technology[J]. Nonferrous Metals Science and Engineering, 2013, 4(1): 26-30.
[25] JADHAV U U, HOCHENG H, WENG W H. Innovative use of biologically produced ferric sulfate for machining of copper metal and study of specific metal removal rate and surface roughness during the process[J]. Journal of Materials Processing Technology, 2013, 213(9): 1509-1515.
[26] ROMERO R, MAZUELOS A, PALENCIA I, CARRANZA F. Copper recovery from chalcopyrite concentrates by the BRISA process[J]. Hydrometallurgy, 2003, 70(1/3): 205-215.
(编辑 王 超)
基金项目:中央重金属污染防治专项资金项目
收稿日期:2014-10-30;修订日期:2015-08-10
通信作者:周洪波,教授,博士;电话:0731-88877216;E-mail: zhouhb@csu.edu.cn
摘 要:利用生物浸出的方法实现废覆铜板分选渣中残留铜的资源化,主要研究Fe2+物质添加量、浸出时间、初始pH和渣投加量(固形物含量)等因素对不同来源废覆铜板渣中铜生物浸出的影响。结果表明:生物浸出铜过程中无需额外再添加Fe2+能源物质且能够短时间内(≤5 h)快速高效浸出不同来源分选残渣中的铜;初始pH和渣投加量对废覆铜板渣中铜浸出产生显著影响。优化结果表明:控制初始pH≤2.2,渣投加量20%~30%,无额外添加酸和Fe2+,两种分选残渣生物浸出5 h后,铜浸出率可达95%以上。
[4] 顾帼华, 戚云峰. 废旧印刷电路板的粉碎性能及资源特征[J]. 中国有色金属学报, 2004, 14(6): 1037-1041.
[6] 王成彦, 邱定蕃, 徐盛明. 金属二次资源循环利用意义, 现状及亟需关注的几个领域[J]. 中国有色金属学报, 2008, 18(1): 359-366.
[11] 龙来寿, 孙水裕, 钟 胜, 刘敬勇, 邓 丰, 李红军. 真空热解预处理对回收废线路板中铜的影响[J]. 中国有色金属学报, 2010, 20(4): 795-800.
[15] 徐 颖, 谢志钢, 薛 璐, 冯岳阳, 陈 玉, 孙 瑛. 氧化亚铁硫杆菌淋滤重金属污染底泥的动力学[J]. 中国有色金属学报, 2014, 24(5): 1352-1358.
[24] 曾伟民, 朱海珍, 叶子捷, 夏明晨, 喻子凌, 王新天. 生物湿法冶金技术回收废弃线路板中有价金属的研究进展[J]. 有色金属科学与工程, 2013, 4(1): 26-30.


